
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Leaf Chromatography Experiment – Easy Paper Chromatography

Leaf chromatography is paper chromatography using leaves. Paper chromatography is a separation technique. When applied to leaves, it separates the pigment molecules mostly according to their size. The main pigment molecule in green leaves is chlorophyll, which performs photosynthesis in the plant. Other pigments also occur, such as carotenoids and anthocyanins. When leaves change color in the fall , the amount and type of pigment molecules changes. Leaf chromatography is a fun science project that lets you see these different pigments.
Leaf Chromatography Materials
You only need a few simple materials for the leaf chromatography project:
- Rubbing alcohol (isopropyl alcohol)
- Coffee filters or thick paper towels
- Small clear jars or glasses with lids (or plastic wrap to cover the jars)
- Shallow pan
- Kitchen utensils
You can use any leaves for this project. A single plant leaf contains several pigment molecules, but for the most colors, use a variety of leaves. Or, collect several of each kind of leaf and compare them to each other. Good choices are colorful autumn leaves or chopped spinach.
Perform Paper Chromatography on Leaves
The key steps are breaking open the cells in leaves and extracting the pigment molecule and then separating the pigment using the alcohol and paper.
- Finely chop 2-3 leaves or several small leaves. If available, use a blender to break open the plant cells. The pigment molecules are in the chloroplasts of the cells, which are organelles encased within the plant cell walls. The more you break up the leave, the more pigment you’ll collect.
- Add enough alcohol to just cover the leaves.
- If you have more samples of leaves, repeat this process.
- Cover the container of leaves and alcohol and set it in a shallow pan filled with enough hot tap water to surround and heat the container. You don’t want water getting into your container of leaves.
- Replace the hot water with fresh water as it cools. Swirl the container of leaves around from time to time to aid the pigment extraction into the alcohol. The extraction is ready when the alcohol is deeply colored. The darker its color, the brighter the resulting chromatogram.
- Cut a long strip of coffee filter or sturdy paper towel for each chromatography jar. Paper with an open mesh (like a paper towel) works quickly, but paper with a denser mesh (like a coffee filter) is slower but gives a better pigment separation.
- Place a strip of paper into jar, with one end in the leaf and alcohol mixture and the other end extending upward and out of the jar.
- The alcohol moves via capillary action and evaporation, pulling the pigment molecules along with it. Ultimately, you get bands of color, each containing different pigments. After 30 to 90 minutes (or whenever you achieve pigment separation), remove the paper strips and let them dry.
How Leaf Chromatography Works
Paper chromatography separates pigments in leaf cells on the basis of three criteria:
- Molecule size
Solubility is a measure of how well a pigment molecule dissolves in the sol vent. In this project, the solvent is alcohol . Crushing the leaves breaks open cells so pigments interact with alcohol. Only molecules that are soluble in alcohol migrate with it up the paper.
Assuming a pigment is soluble, the biggest factor in how far it travels up the paper is particle size. Smaller molecules travel further up the paper than larger molecules. Small molecules fit between fibers in the paper more easily than big ones. So, they take a more direct path through the paper and get further in less time. Large molecules slowly work their way through the paper. In the beginning, not much space separates large and small molecules. The paper needs to be long enough that the different-sized molecules have enough time to separate enough to tell them apart.
Paper consists of cellulose, a polysaccharide found in wood, cotton, and other plants. Cellulose is a polar molecule . Polar molecules stick to cellulose and don’t travel very far in paper chromatography. Nonpolar molecules aren’t attracted to cellulose, so they travel further.
Of course, none of this matters if the solvent doesn’t move through the paper. Alcohol moves through paper via capillary action . The adhesive force between the liquid and the paper is greater than the cohesive force of the solvent molecules. So, the alcohol moves, carrying more alcohol and the pigment molecules along with it.
Interpreting the Chromatogram
- The smallest pigment molecules are the ones that traveled the greatest distance. The largest molecules are the ones that traveled the least distance.
- If you compare chromatograms from different jars, you can identify common pigments in their leaves. All things being equal, the lines made by the pigments should be the same distance from the origin as each other. But, usually conditions are not exactly the same, so you compare colors of lines and whether they traveled a short or long distance.
- Try identifying the pigments responsible for the colors.
There are three broad classes of plant pigments: porphyrins, carotenoids, and flavonoids. The main porphyrins are chlorophyll molecules. There are actually multiple forms of chlorophyll, but you can recognize them because they are green. Carotenoids include carotene (yellow or orange), lycopene (orange or red), and xanthophyll (yellow). Flavonoids include flavone and flavonol (both yellow) and anthocyanin (red, purple, or even blue).
Experiment Ideas
- Collect leaves from a single tree or species of tree as they change color in the fall. Compare chromatograms from different colors of leaves. Are the same pigments always present in the leaves? Some plants produce the same pigments, just in differing amounts. Other plants start producing different pigments as the seasons change.
- Compare the pigments in leaves of different kinds of trees.
- Separate leaves according to color and perform leaf chromatography on the different sets. See if you can tell the color of leaves just by looking at the relative amount of different pigments.
- The solvent you use affects the pigments you see. Repeat the experiment using acetone (nail polish remover) instead of alcohol.
- Block, Richard J.; Durrum, Emmett L.; Zweig, Gunter (1955). A Manual of Paper Chromatography and Paper Electrophoresis . Elsevier. ISBN 978-1-4832-7680-9.
- Ettre, L.S.; Zlatkis, A. (eds.) (2011). 75 Years of Chromatography: A Historical Dialogue . Elsevier. ISBN 978-0-08-085817-3.
- Gross, J. (1991). Pigments in Vegetables: Chlorophylls and Carotenoids . Van Nostrand Reinhold. ISBN 978-0442006570.
- Haslam, Edwin (2007). “Vegetable tannins – Lessons of a phytochemical lifetime.” Phytochemistry . 68 (22–24): 2713–21. doi: 10.1016/j.phytochem.2007.09.009
- McMurry, J. (2011). Organic chemistry With Biological Applications (2nd ed.). Belmont, CA: Brooks/Cole. ISBN 9780495391470.
Related Posts
Your browser is not supported
Sorry but it looks as if your browser is out of date. To get the best experience using our site we recommend that you upgrade or switch browsers.
Find a solution
- Skip to main content
- Skip to navigation

- Back to parent navigation item
- Primary teacher
- Secondary/FE teacher
- Early career or student teacher
- Higher education
- Curriculum support
- Literacy in science teaching
- Periodic table
- Interactive periodic table
- Climate change and sustainability
- Resources shop
- Collections
- Remote teaching support
- Starters for ten
- Screen experiments
- Assessment for learning
- Microscale chemistry
- Faces of chemistry
- Classic chemistry experiments
- Nuffield practical collection
- Anecdotes for chemistry teachers
- On this day in chemistry
- Global experiments
- PhET interactive simulations
- Chemistry vignettes
- Context and problem based learning
- Journal of the month
- Chemistry and art
- Art analysis
- Pigments and colours
- Ancient art: today's technology
- Psychology and art theory
- Art and archaeology
- Artists as chemists
- The physics of restoration and conservation
- Ancient Egyptian art
- Ancient Greek art
- Ancient Roman art
- Classic chemistry demonstrations
- In search of solutions
- In search of more solutions
- Creative problem-solving in chemistry
- Solar spark
- Chemistry for non-specialists
- Health and safety in higher education
- Analytical chemistry introductions
- Exhibition chemistry
- Introductory maths for higher education
- Commercial skills for chemists
- Kitchen chemistry
- Journals how to guides
- Chemistry in health
- Chemistry in sport
- Chemistry in your cupboard
- Chocolate chemistry
- Adnoddau addysgu cemeg Cymraeg
- The chemistry of fireworks
- Festive chemistry
- Education in Chemistry
- Teach Chemistry
- On-demand online
- Live online
- Selected PD articles
- PD for primary teachers
- PD for secondary teachers
- What we offer
- Chartered Science Teacher (CSciTeach)
- Teacher mentoring
- UK Chemistry Olympiad
- Who can enter?
- How does it work?
- Resources and past papers
- Top of the Bench
- Schools' Analyst
- Regional support
- Education coordinators
- RSC Yusuf Hamied Inspirational Science Programme
- Science Education Policy Alliance
- RSC Education News
- Supporting teacher training
- Interest groups

- More navigation items
Leaf chromatography
In association with Nuffield Foundation
- Five out of five
- No comments
Try this class practical using paper chromatography to separate and investigate the pigments in a leaf
Most leaves are green due to chlorophyll. This substance is important in photosynthesis (the process by which plants make their food). In this experiment, students investigate the different pigments present in a leaf, from chlorophyll to carotenes, using paper chromatography.
The experiment takes about 30 minutes and can be carried out in groups of two or three students.
- Eye protection
- Pestle and mortar
- Chromatography paper
- Beaker, 100 cm 3
- Small capillary tube (see note 1)
- Cut-up leaves, or leaves and scissors (see note 2)
- Propanone (HIGHLY FLAMMABLE, IRRITANT), supplied in a small bottle fitted with a teat pipette (see note 3)
Equipment notes
- The capillary tubing can be ‘home-made’ from lengths of ordinary glass tubing (diameter: 3–4 mm) using a Bunsen burner fitted with a flame-spreading (‘fish-tail’) jet.
- A variety of leaves can be used. Best results are obtained from trees or bushes with dark green leaves, eg holly.
- Preferably use teat pipettes that do not allow squirting, eg those fitted to dropper bottles of universal indicator.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Propanone, CH 3 COCH 3 (l), (HIGHLY FLAMMABLE, IRRITANT) – see CLEAPSS Hazcard HC085A . The vapour of propanone is HIGHLY FLAMMABLE. Do not have any source of ignition nearby.
- Finely cut up some leaves and fill a mortar to about 2 cm depth.
- Add a pinch of sand and about six drops of propanone from the teat pipette.
- Grind the mixture with a pestle for at least three minutes.
- On a strip of chromatography paper, draw a pencil line 3 cm from the bottom.
- Use a fine glass tube to put liquid from the leaf extract onto the centre of the line. Keep the spot as small as possible.
- Allow the spot to dry, then add another spot on top. Add five more drops of solution, letting each one dry before putting on the next. The idea is to build up a very concentrated small spot on the paper.
- Attach the paper to the pencil using sellotape so that when placed in the beaker, the paper is just clear of its base.
- Place no more than about 10 cm 3 of propanone in the beaker and hang the paper so it dips in the propanone. Ensure the propanone level is below the spot.
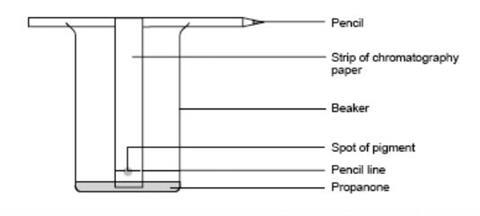
Source: Royal Society of Chemistry
The equipment required for using paper chromatography to separate the different pigments in leaves
- Avoid moving the beaker in any way once the chromatography has started.
- Leave the experiment until the propanone has soaked near to the top, and then remove the paper from the beaker.
- Mark how high the propanone gets on the paper with a pencil and let the chromatogram dry.
Teaching notes
This experiment works very well providing care is taken over preparing the spot on the chromatography paper. It should be as small and as concentrated as possible. Encourage students to be patient and to wait until each application is dry before adding the next.
At least three spots should be obtained, and one of these should be yellow due to carotenes.
The extent to which any particular component moves up the paper is dependent not only on its solubility in propanone but also on its attraction for the cellulose in the chromatography paper. The yellow carotene spot (with a higher RF value) tends to move up the paper the furthest.
More resources
Add context and inspire your learners with our short career videos showing how chemistry is making a difference .
Additional information
This is a resource from the Practical Chemistry project , developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology .
© Nuffield Foundation and the Royal Society of Chemistry
- 11-14 years
- 14-16 years
- Practical experiments
- Chromatography
Specification
- 2. Develop and use models to describe the nature of matter; demonstrate how they provide a simple way to to account for the conservation of mass, changes of state, physical change, chemical change, mixtures, and their separation.
- Chromatography as a separation technique in which a mobile phase carrying a mixture is caused to move in contact with a selectively absorbent stationary phase.
- 6 Investigate how paper chromatography can be used to separate and tell the difference between coloured substances. Students should calculate Rf values.
- Chromatography involves a stationary phase and a mobile phase. Separation depends on the distribution of substances between the phases.
- The ratio of the distance moved by a compound (centre of spot from origin) to the distance moved by the solvent can be expressed as its Rf value: Rf = (distance moved by substance / distance moved by solvent)
- Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These physical processes do not involve chemical reactions and no new substances are made.
- Recall that chromatography involves a stationary and a mobile phase and that separation depends on the distribution between the phases.
- Interpret chromatograms, including measuring Rf values.
- Suggest chromatographic methods for distinguishing pure from impure substances.
- 12 Investigate how paper chromatography can be used to separate and tell the difference between coloured substances. Students should calculate Rf values.
- 2.11 Investigate the composition of inks using simple distillation and paper chromatography
- 2.9 Describe paper chromatography as the separation of mixtures of soluble substances by running a solvent (mobile phase) through the mixture on the paper (the paper contains the stationary phase), which causes the substances to move at different rates…
- C2.1g describe the techniques of paper and thin layer chromatography
- 2.9 Describe paper chromatography as the separation of mixtures of soluble substances by running a solvent (mobile phase) through the mixture on the paper (the paper contains the stationary phase), which causes the substances to move at different rates o…
- C5.1.4 recall that chromatography involves a stationary and a mobile phase and that separation depends on the distribution between the phases
- 3 Using chromatography to identify mixtures of dyes in a sample of an unknown composition
- C3 Using chromatography to identify mixtures of dyes in a sample of an unknown composition
- 1.9.5 investigate practically how mixtures can be separated using filtration, crystallisation, paper chromatography, simple distillation or fractional distillation (including using fractional distillation in the laboratory to separate miscible liquids…
- 1.9.7 interpret a paper chromatogram including calculating Rf values;
- carry out paper and thin-layer chromatography and measure the Rf values of the components and interpret the chromatograms;
Related articles

Solubility | Review my learning worksheets | 14–16 years
By Lyn Nicholls
Identify learning gaps and misconceptions with this set of worksheets offering three levels of support

How to teach chromatography at post-16
2024-03-11T04:00:00Z By Andy Markwick
Everything you need to help your students master the fundamentals of this analytical technique

Chromatography challenge | 16–18 years
By Andy Markwick
Explore analytical techniques and their applications with a chromatography investigation and research activity
No comments yet
Only registered users can comment on this article., more experiments.

‘Gold’ coins on a microscale | 14–16 years
By Dorothy Warren and Sandrine Bouchelkia
Practical experiment where learners produce ‘gold’ coins by electroplating a copper coin with zinc, includes follow-up worksheet

Practical potions microscale | 11–14 years
By Kirsty Patterson Four out of five
Observe chemical changes in this microscale experiment with a spooky twist.


Antibacterial properties of the halogens | 14–18 years
By Kristy Turner
Use this practical to investigate how solutions of the halogens inhibit the growth of bacteria and which is most effective
- Contributors
- Email alerts
Site powered by Webvision Cloud
Separation of Plant Pigments by Paper Chromatography
The separation of plant pigments by paper chromatography is an analysis of pigment molecules of the given plant. Chromatography refers to colour writing . This method separates molecules based on size, density and absorption capacity.
Chromatography depends upon absorption and capillarity . The absorbent paper holds the substance by absorption. Capillarity pulls the substance up the absorbent medium at different rates.
Separated pigments show up as coloured streaks . In paper chromatography, the coloured bands separate on the absorbent paper. Chlorophylls, anthocyanins, carotenoids, and betalains are the four plant pigments.
This post discusses the steps of separating plant pigments through paper chromatography. Also, you will get to know the observation table and the calculation of the Rf value.
Content: Separation of Plant Pigments by Paper Chromatography
Paper chromatography, plant pigments, steps of plant pigment separation, observation, calculation.
It is the simplest chromatography method given by Christian Friedrich Schonbein in 1865. Paper chromatography uses filter paper with uniform porosity and high resolution.
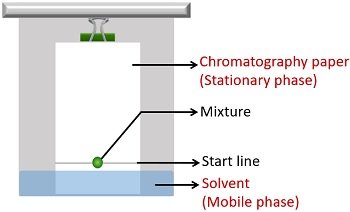
The mixtures in compounds have different solubilities . For this reason, they get separated distinctly between the stationary and running phase.
- The mobile phase is a combination of non-polar organic solvents. The solvent runs up the stationary phase via capillary movement.
- The stationary phase is polar inorganic solvent, i.e. water. Here, the absorbent paper supports the stationary phase, i.e. water.
Plant pigments are coloured organic substances derived from plants. Pigments absorb visible radiation between 380 nm (violet) and 760 nm (red).
They give colour to stems, leaves, flowers, and fruits. Also, they regulate processes like photosynthesis, growth, and development.
Plants produce various forms of pigments. Based on origin, function and water solubility, plant pigments are grouped into:
- Chlorophylls (green)
- Carotenoids (yellow, orange-red)
- Anthocyanins (red to blue, depending on pH)
- Betalains (red or yellow)
Chlorophyll : It is a green photosynthetic pigment. Chlorophyll a and b are present within the chloroplasts of plants. Because of the phytol side chain, they are water-repelling . Their structure resembles haemoglobin. But, they contain magnesium as a central metal instead of iron.
Carotenoids : These are yellow to yellow-orange coloured pigments. Also, they are very long water-repelling pigments. Carotenoids are present within the plastids or chromoplasts of plants.
Anthocyanins : These appear as red coloured pigments in vacuoles of plant cells. Anthocyanins are water-soluble pigments. They give pink-red colour to the petals, fruits and leaves.
Betalains : These are tyrosine derived water-soluble pigments in plants. Betacyanins (red-violet) and betaxanthins (yellow-orange) are the two pigments coming in this category. They are present in vacuoles of plant cells.
You can separate all the above pigments using paper chromatography.
Video: Separation of Plant Pigments
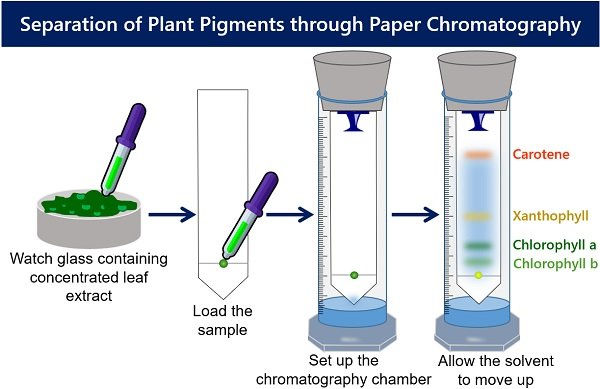
Preparation of Concentrated Leaf Extract
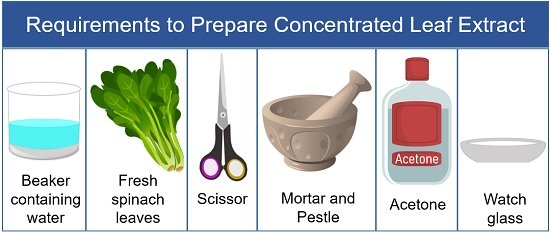
- Wash spinach leaves in distilled water.
- Then take out the spinach leaves and allow the moisture to dry out.
- After that, take a scissor and cut the leaves into the mortar.
- Take a little volume of acetone into the mortar. Note : Acetone is used instead of water to mash the leaves because it is less polar than the water. This allows a high resolution of the molecules in the sample between the absorbent paper.
- Then, grind spinach leaves using a pestle until liquid paste forms. Note : The liquid in the crushed leaf paste is the pigment extract.
- After that, take out the mixture into the watch glass or Petri dish.
Load the Leaf Extract onto Absorbent Paper

- Take Whatman filter paper and draw a line above 2 cm from the bottom margin. You can use a pencil and scale to draw a fainted line. Note : A pencil is used because pencil marks are insoluble in the solvent.
- Then, cut the filter paper to make a conical edge from the line drawn towards the margin end. You can use a scissor to cut the Whatman filter paper. Note : The conical end at the bottom of the filter paper results in better separation.
- Put a drop of leaf extract on the centre of a line drawn on the absorbent paper.
- Then, at the same time dry the absorbent paper.
- Repeat the above two steps many times so that the spot becomes concentrated enough.
Setup the Chromatography Chamber
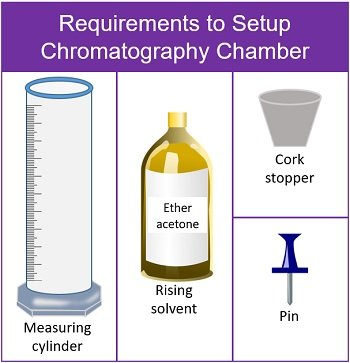
- Take a clean measuring cylinder and add rising solvent (ether acetone) up to 4 ml.
- Bend the strip of paper from the top. Then, using a pushpin attach the paper to the bottom of the cork.
- Adjust the length of the paper. The absorbent paper should not touch the surface of the measuring cylinder.
- After that, allow the solvent to move up the absorbent paper.
- When the solvent front has stopped moving, remove the paper.
- Allow it to dry for a while until the colours completely elute from the paper.
- At last, mark the front edge travelled by each pigment.
Over the dried paper strip, you will see four different bands. Different colour streaks form because of different affinities with the mobile phase (solvent).
- The carotene pigment appears at the top as a yellow-orange band.
- A yellowish band appears below the carotene, which indicates xanthophyll pigment.
- Then a dark green band represents the chlorophyll-a pigment.
- The chlorophyll-b pigment appears at the bottom as a light green band.
Observation Table
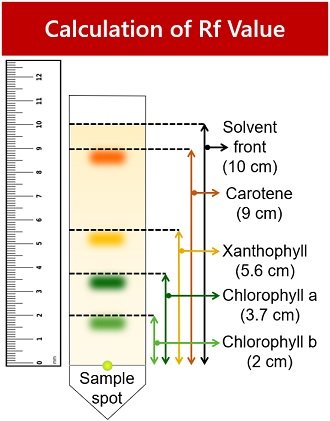
1. Light green spot indicates chlorophyll-b pigment.
- Rf value= Distance chlorophyll-b travelled / Distance solvent travelled = 2/10 = 0.2
2. Dark green spot represents chlorophyll-a pigment.
- Rf value= Distance chlorophyll-a travelled / Distance solvent travelled = 3.7/10 = 0.37
3. The yellow band represents xanthophyll pigment.
- Rf value= Distance xanthophyll travelled / Distance solvent travelled = 5.6/10 = 0.56
4. The yellow-orange band indicates carotene pigment.
- Rf value= Distance carotene travelled / Distance solvent travelled = 9/10 = 0.9
Factors affecting the Rf values of a particular analyte are:
- Stationary phase
- The concentration of the stationary phase
- Mobile phase
- The concentration of the mobile phase
- Temperature
The Rf value of compounds in the mixture differs by any changes in the concentration of stationary and mobile phases.
Temperature affects the solvent capillary movement and the analyte’s solubility in the solvent. Rf value is independent of the sample concentration. Its value is always positive .
Related Topics:
- Difference Between Budding and Grafting
- Phototropism in Plants
- Potometer Experiment
1 thought on “Separation of Plant Pigments by Paper Chromatography”
Nice experiment and understanding.
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Start typing and press enter to search
Talk to our experts
1800-120-456-456
Separation Of Plant Pigments Through Paper Chromatography

CBSE Biology Experiment- Separation of Plant Pigments Through Paper Chromatography
A plant contains several pigments that enable its metabolic capabilities. Chlorophyll, of course, is the most important plant pigment in the leaves of photosynthetic plants. It is what gives most plants their characteristic green colour and mediates the processes of photosynthesis as well. Besides chlorophyll, plants contain a group of pigments called carotenoids, yellow-orange pigments. Xanthophyll and carotene are the two most important carotenoids. How does one separate these pigments of a plant from visualising their colour? Carry out this simple yet interesting experiment to find out!
Table of Content
Apparatus required
Observations
Precautions
To separate and visualise plant pigments present in spinach leaf using the technique of paper chromatography
Apparatus Required
Spinach leaves, pestle and mortar, water, chromatography jar, ether acetone solvent, scissors, Whatman No.1 filter paper strips, watch glass, capillary tube, pencil, scale
A typical photosynthetic plant contains several pigments across its stems, leaves and flowers.
The leaves of a photosynthetic plant contain the photosynthetic pigments chlorophyll a and chlorophyll b, which impart green colour to the plant, and are contained in the chloroplasts.
They form the reaction centres of photosynthesis by absorbing the incident sunlight.
The second most commonly occurring plant pigments are the carotenoids- namely, xanthophyll and carotene. The carotenoids are the yellow-orange pigments of the plant, present within chromoplasts.
The different pigments occurring in the leaves of a plant can be visualised using paper chromatography.
Principles of Paper Chromatography
Martin and Synge developed paper chromatography in the year 1943. It is a simple technique used to visualise different components within a mixture.
Paper chromatography is based on the different solubilities of the various components of a mixture in a particular solvent. Based on their solubilities in the solvent, each component is separated.
Paper chromatography comprises two phases- the stationary phase, which is a nonpolar medium of cellulose and water, and the mobile phase , which is a polar, organic solvent (e.g. ether acetone solvent)
The mixture is loaded onto the paper, which is then suspended in an appropriate solvent (the mobile phase)
Absorption, solubility and capillarity are the three factors that affect the separation of the components and the distance travelled by each component from the initial spot.
Absorption holds the substance onto the paper. Capillary action pulls up the substances through the paper. Different solubilities separate the components in the mixture.
The most soluble and lighter substance moves farthest, while the least soluble and heavier substance separates early on near the loading point on the paper
The separated pigments appear as streaks at different positions on the chromatography paper.
Take a few fresh spinach leaves and wash them under distilled water
Using your scissors, cut the spinach leaves into small pieces and add them into the mortar, along with a few drops of acetone (~5mL). Grind into a fine paste
Take a strip of Whatman filter paper No.1 (6” x 1.5”) and cut one end such that it has a tapering (arrowed) notch
Using a pencil, mark a horizontal line 2 cm above the tip of the notch
Add just one drop of the prepared leaf paste onto the centre of this horizontal line using a capillary tube
Once the drop dries, add another drop of the sample onto the same spot
Repeat steps 5-6 at least 4-5 times. This will ensure a concentrated sample.
Add ~5mL of the ether acetone solvent into the chromatography jar
Now suspend the loaded filter paper onto the hook of the cork of the chromatography jar. For this step, one can also use a measuring cylinder, a cork, and a pushpin.
The pointed end of the paper should just touch the solvent once suspended. The horizontal line marked on the paper MUST NOT TOUCH the solvent
Allow the solvent to rise through the filter paper. This may take up to 40 minutes.
Once the pigments have separated into four distinct bands, and the solvent has stopped rising, remove the paper from the hook.
Mark the edge of the solvent on the paper. This is the solvent front.
Allow the paper to dry completely.
Observations
The chromatogram displays four distinct bands of colour, indicating the separation of the leaf pigments.
The pigments within the mixture have separated into four distinct bands as follows, from the bottom up to the solvent front:
Band of green colour- chlorophyll b
Band of blue-green colour- chlorophyll a
Band of yellow colour- xanthophyll
Band of orange colour- carotene
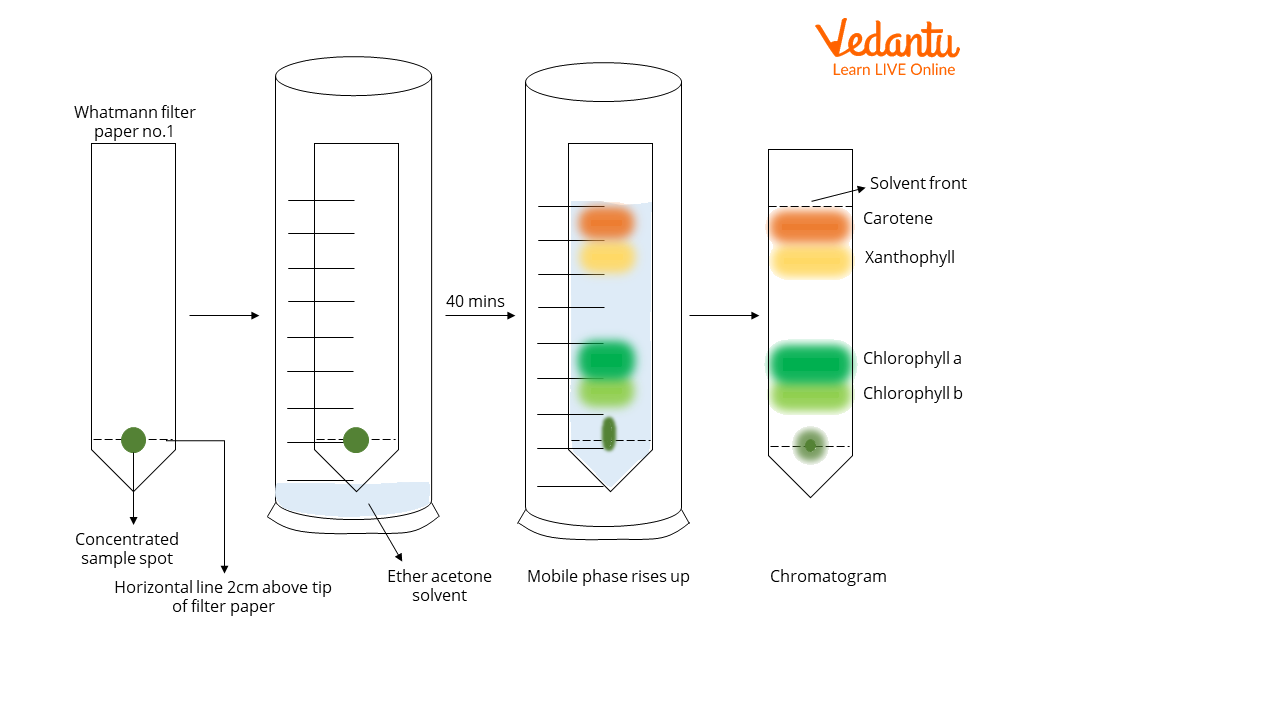
Separation of leaf pigments using paper chromatography
Do not use a ball or ink pen to mark the chromatography paper.
Ensure that the loaded spot is not touching the solvent and is visible above it
Make sure to transfer a generous amount of the sample onto the paper to ensure better results
Be careful not to handle ether acetone near any flame source, as this solvent is highly flammable.
Lab Manual Questions
1. Which photosynthetic pigment travels the farthest and why?
Ans: Carotene travels the farthest, near the solvent front. This is because it is more soluble in the solvent than the other three pigments and doesn’t bond to the medium of the paper via hydrogen bonds.
2. Why is acetone used as a solvent instead of water?
Ans: Acetone is less polar than water. This allows for greater allows greater resolution, i.e., better separation of the pigments in the mixture
3. What will happen if the spot touches the solvent?
Ans: It is important to ensure that the sample doesn’t touch the mobile phase. If it does, the mixture spot-loaded will dissolve immediately in the solvent, thereby maligning the entire experiment
Viva Questions
1. How many pigments have you separated from the sample? What are they?
Ans: Four pigments have been separated from the sample, chlorophyll a, chlorophyll b, xanthophyll and carotene
2. Why do you think acetone is used as the mobile phase in this experiment, not water?
Ans: Ether - acetone is a non-polar solvent. The natural pigments present in the leaves are, however, polar. This is important since separation in paper chromatography is based on the differing solubilities of the pigments in the solvent and the stationary phase.
3. Based on your experiment, what can you infer about the polarities of the separated pigments?
Ans: The most polar pigment is chlorophyll b, followed by chlorophyll a, xanthophyll, and finally, carotene, which is the least polar, hence most soluble in the non-polar solvent.
4. What do you know about the polarity of the chromatography paper?
Ans: The chromatography paper is made up of cellulose, attracting water vapour molecules, thereby contributing to the polar nature of the paper.
5. How many different types of pigments occur in plants? Can you name these pigments?
Ans: Four types of pigments occur in plants- the chlorophylls, and the carotenoids, which include carotene and xanthophylls like lutein, anthocyanin, and betalains.
6. We are well aware that chlorophylls are involved in photosynthesis. What are the functions of carotenoids?
Ans: Carotenoids are also involved in photosynthesis. They absorb wavelengths not received by chlorophylls and transfer the absorbed energy to the chlorophyll molecules. Hence, they are also called accessory pigments. They are also involved in photoprotection.
7. Apart from paper chromatography, what other types of chromatographies are used for separation purposes?
Ans: Thin layer chromatography, column chromatography, gas chromatography, ion-exchange chromatography, affinity chromatography, etc., are the other types used to separate components in a mixture.
8. What are the absorption spectrum values of chlorophyll a and b?
Ans: Chlorophyll a absorbs the maximum wavelength of 642 nm and 372nm (red and blue regions, respectively), while chlorophyll b absorbs a maximum wavelength of 626 nm and 372 nm (red and blue regions, respectively).
Practical Based Questions
The pigment that appears blue-green on the chromatogram is?
Chlorophyll a
Chlorophyll b
Xanthophyll
Carotene
Ans. A. Chlorophyll a
The pigment that is most soluble in the solvent is:
Ans. D. Carotene
The stationary phase employed in the paper chromatography experiment is:
Ether-acetone
Ans. D. Paper
Which of the following is not involved in separating leaf pigments in paper chromatography?
Solubility
Capillarity
Gravitation
Ans. D. Gravitation
Xanthophyll belongs to _____ group of plant pigments
Chlorophylls
Carotenoids
Ans. C. Carotenoids
The P700 reaction centre comprises
More of chlorophyll a than chlorophyll b
More of chlorophyll b than chlorophyll a
Equal amounts of chlorophyll a
Only chlorophyll a
Ans. A. More of chlorophyll a than chlorophyll b
The pigment that is least soluble in the mobile phase is
Ans. B. Chlorophyll b
The pigment that travels farthest on the paper is
Most soluble in the solvent
Least soluble in the solvent
Not soluble in the solvent
Of lower molecular weight than the other pigments
Ans. A. Most soluble in the solvent
A leaf from a typical photosynthetic plant comprises four pigments: chlorophyll a and b, xanthophyll and carotene.
These pigments can be separated using the paper chromatography technique, using acetone as the mobile phase/ solvent.
Four bands of separated pigments are observed, with chlorophyll b at the bottom, followed by chlorophyll a, xanthophyll and finally, carotene at the top.
FAQs on Separation Of Plant Pigments Through Paper Chromatography
1. What is the principle behind the technique of paper chromatography?
Paper chromatography is based on the principle of partition of the substance between the two phases-i.e., the mobile phase (acetone solvent) and the stationary phase (paper). The partition is affected by the relative solubility of the mixture's components between the two phases.
2. What material is used for the chromatography paper? Why?
The chromatography paper is made up of cellulose, a polar substance. This is used because the pigments in the mixture will be separated based on their polarity and solubility in the non-polar solvent.
3. What is the retention factor in paper chromatography?
The retention factor (R f ) is the ratio of the distance travelled by the solute on the chromatography paper to the distance travelled by the solvent on the paper. This ratio remains unchanged for a particular solute when the stationary and mobile phases are the same.


IMAGES
COMMENTS
Sep 28, 2021 · Leaf chromatography is paper chromatography using leaves. Paper chromatography is a separation technique. When applied to leaves, it separates the pigment molecules mostly according to their size. The main pigment molecule in green leaves is chlorophyll, which performs photosynthesis in the plant.
In this experiment, students investigate the different pigments present in a leaf, from chlorophyll to carotenes, using paper chromatography. The experiment takes about 30 minutes and can be carried out in groups of two or three students. Equipment Apparatus. Eye protection; Pestle and mortar; Chromatography paper; Beaker, 100 cm 3
Let us discuss the procedure of separating plant pigments by paper chromatography. The diagram below indicates the major steps of pigment separation. First, the concentrated leaf extract is prepared. After that, load the sample on the chromatography paper. Then, keep a paper containing sample spot inside the chromatography chamber containing ...
1. Cut a piece of Whatman #1 filter paper or chromatography paper to the dimensions of 12 cm X 14 cm. Edges must be straight. 2. With a pencil lightly make a line 1.5 - 2 cm from the bottom edge of the paper which measures 14 cm. 3. Select 2 large dark green spinach leaves and blot dry with paper towels. Place a leaf over the
The video gives an overview of what paper chromatography is, shows how it is done, explains the separation processes involved, and also provides tips and tricks for troubleshooting your experiment. In this project, you will be using paper chromatography to investigate chlorophyll and other pigments in plant leaves.
1. Take a strip of chromatography paper approximately 18 cm long. One end is blunt and the other is pointed. 2. With a pencil lightly make a line 2 cm from the pointed end of the paper. 3. Bend the strip of paper at the blunt end and attach it to the small end of the cork with the push pin. Adjust the length of the paper so that when it is
CHLOROPHYLL EXTRACTION Analysis: Paper chromatography Sketch your paper chromatography observations on the filter paper diagram below. Questions: Paper chromatography . 1. Both chlorophyll-a and chlorophyll-b are present in spinach leaves. What evidence supports this statement, and how can you tell each type of chlorophyll apart? 2.
The process of paper chromatography is also the same as the leaf chromatography experiment. Separation of Chlorophyll Pigments by Paper Chromatography. The chlorophyll molecule is present in the leaf and can be separated by using paper chromatography. The paper chromatography separates the pigments in the leaf based on the distance travelled by ...
The different pigments occurring in the leaves of a plant can be visualised using paper chromatography. Principles of Paper Chromatography. Martin and Synge developed paper chromatography in the year 1943. It is a simple technique used to visualise different components within a mixture. Paper chromatography is based on the different ...
paper as it is absorbed into the paper fibers. origin (where you apply the pigment extract) the “solvent front” is the position of the liquid solvent on the chromatography paper at any given time. the solvent will gradually move from the bottom toward the top of the paper, carrying dissolved pigments with it. stop the chromatogram