- No category

Biology – Potatoes Osmosis Lab Report

Related documents

Study collections
- education if my future
Add this document to collection(s)
You can add this document to your study collection(s)
Add this document to saved
You can add this document to your saved list
Suggest us how to improve StudyLib
(For complaints, use another form )
Input it if you want to receive answer

Potato Osmosis Lab
I have taken this classic biology lab activity illustrating the principles of diffusion and osmosis and adapted it as an online activity. I did this lab many times with my 10th grade regular bio class at Kelly High School in Chicago, but it can be used successfully with kids ranging from middle school to AP Bio. Students can read through the background here and make their own graphs, analyze these data, and draw conclusions.
-Aaron Reedy

Molecules are constantly in motion as a result of a cell's stored kinetic energy, which causes them to bump into each other and move in random new directions. Diffusion is the movement of molecules from an area of where there are many (high concentration) to an area where there are fewer (low concentration). Osmosis is the diffusion of water through a semipermeable membrane. It is important to remember that a semipermeable membrane allows the solvent (usually water) to pass through, but restricts the movement of a solute (a thing dissolved in the solvent). Water will move across a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration.
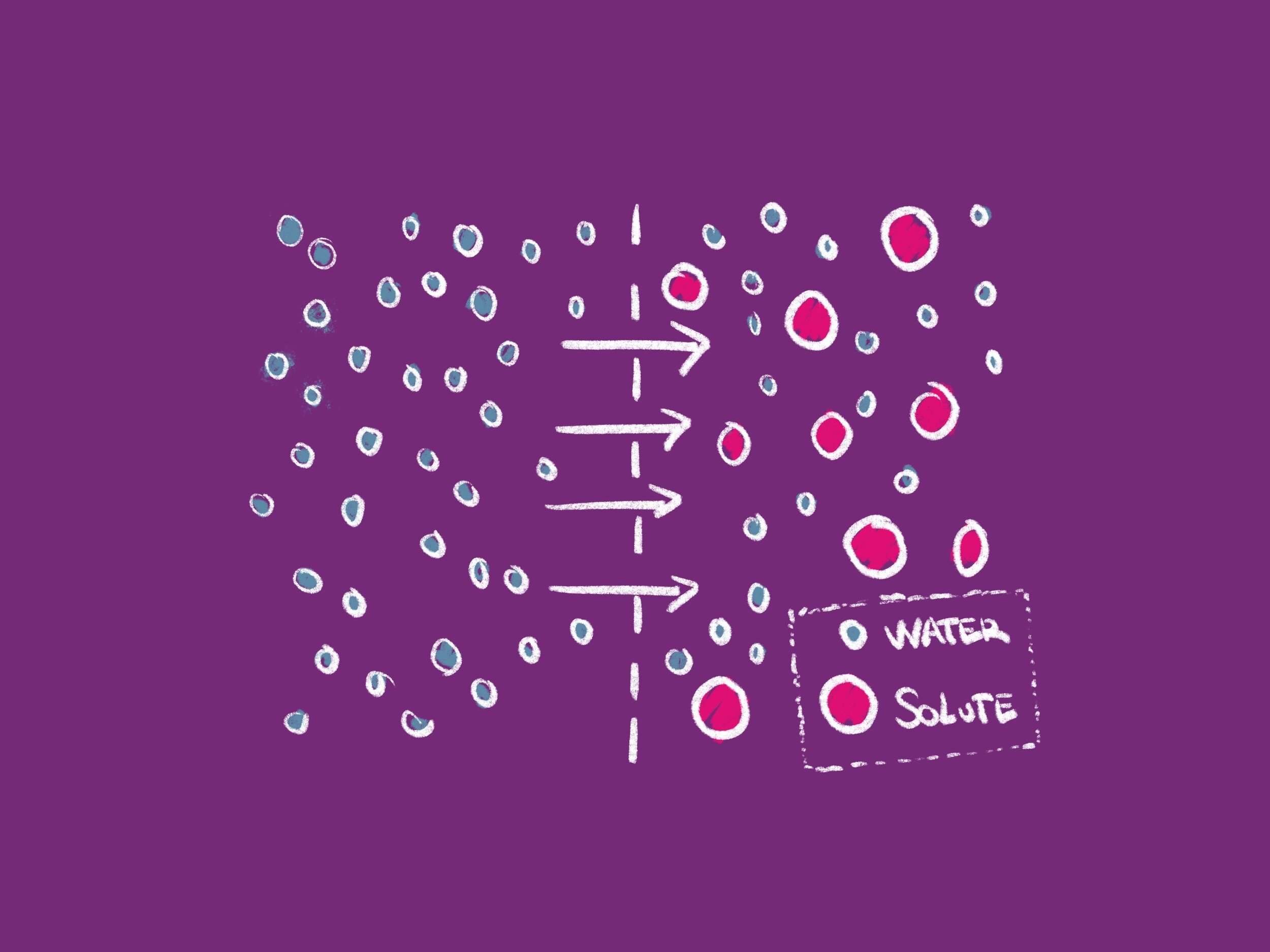
When each side of a membrane has equal solute concentration, the solution is said to be isotonic and water molecules will be equally likely to move in both directions across the membrane. In the case of a hypertonic solution, there is more solute outside the cell than inside the cell. Hypertonic solutions cause water molecules to move out of the cell and into the region of higher solute concentration. Conversely, in hypotonic solutions there is a higher solute concentration inside the cell than outside, and water molecules move into the cell. Whenever possible, water will always move from an area of high water concentration/low solute concentration to an area of low water concentration/high solute concentration.
In this activity, we are going to explore osmosis by looking at a dataset produced with a classic classroom experiment. The experiment uses pieces of potato that are placed in six different solutions of water each with a different solute concentration. The solute is sucrose and the concentrations are measured in units of molarity. The solutions range from no solute to a high concentration of solute and are 0.0 (distilled water), 0.2, 0.4, 0.6, 0.8 and 1.0 molar sucrose.
Pieces of potato are cut to similar sizes, weighed, and then placed in one of the six solutions overnight. The next day, the potato pieces are removed from the solutions, blotted dry, and their final masses are recorded.
Each row in this tidy dataset contains an observation for a single potato piece. Each column in the dataset is a variable and the cells in that column are the values of that variable. The variables recorded for each potato piece are Lab Group Name, Sucrose Concentration (Molarity), Initial Mass (g), Final Mass (g), and Mass Change (%).
To see a video clearly illustrating and explaining the general procedure for this lab, watch Paul Andersen’s Bozeman Science video walkthrough:
The activity
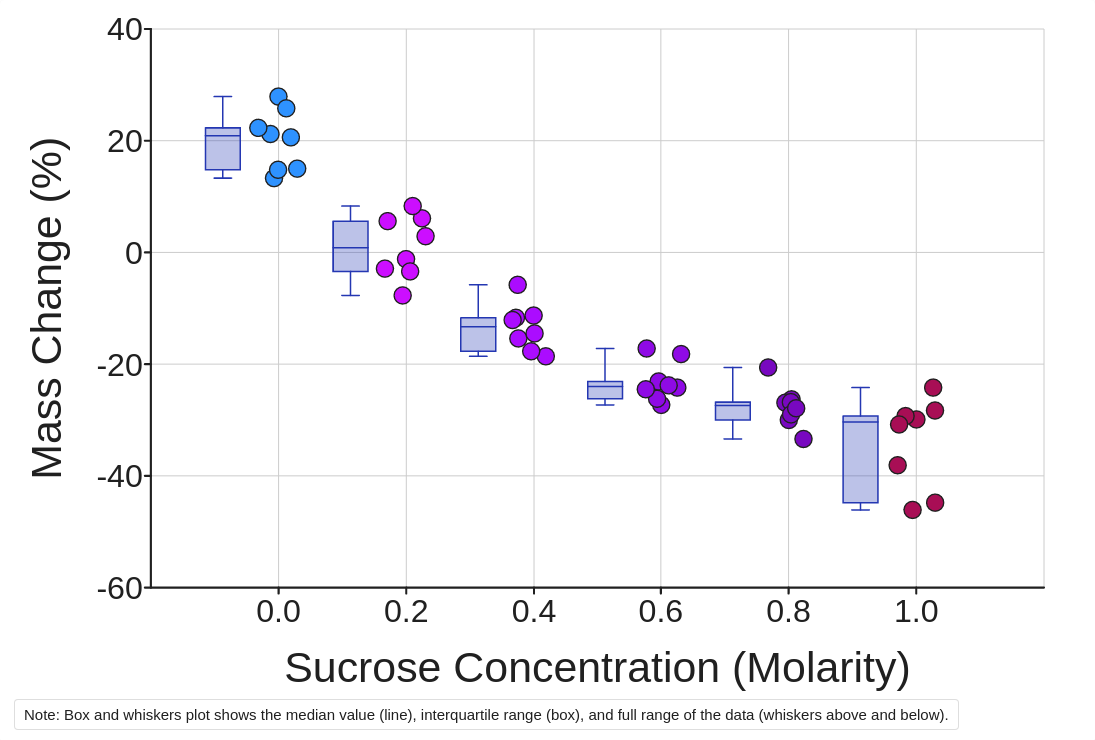
1. Click the yellow Make a graph button to visualize your data. Choose the scatter plot icon and Show Sucrose Concentration (Molar) on the X-axis and Mass Change (%) on the Y-axis. You can add descriptive statistics like means and medians by checking the box just to the right of the graph.
Observe patterns in the data:
2. What are the independent and dependent variables in this experiment?
3. How does Change in Mass (%) change with Sucrose Concentration (Molarity)?
4. Which substance moved across the cellular membrane in this activity? What is the specific name of the movement in terms of this substance?
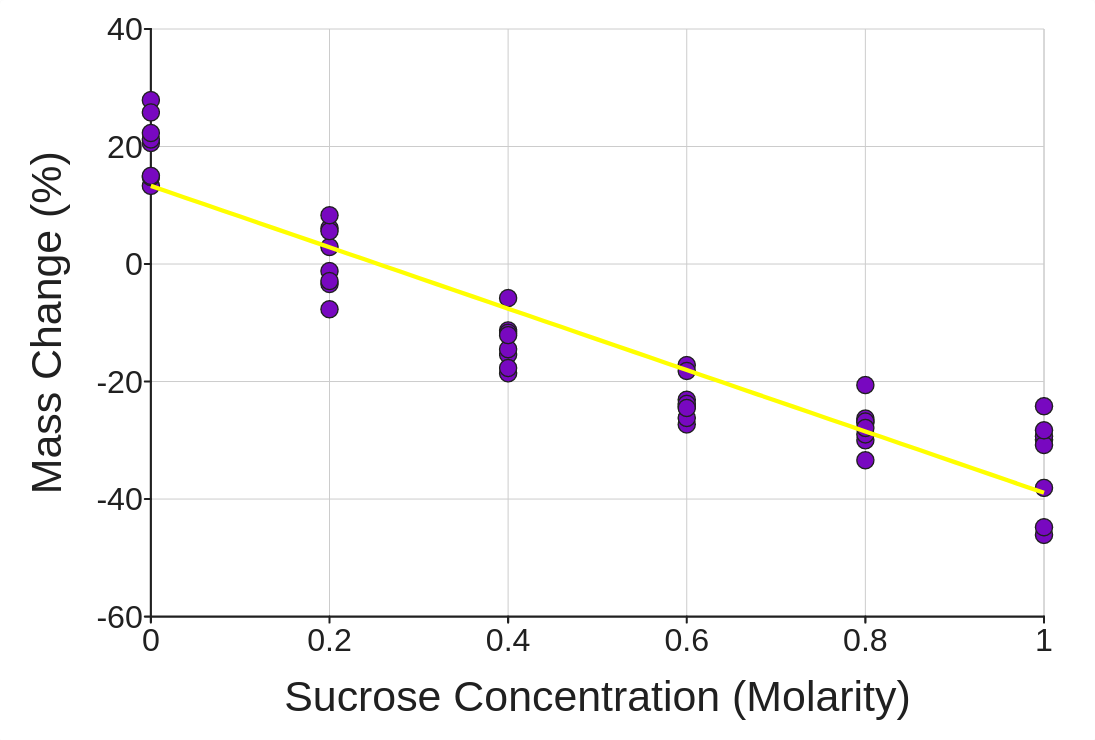
5. Now, change the variable called Sucrose Concentration (Molarity) to a Numeric variable with the dropdown menu right below the variable name near the top of the page. Then add it back to the graph again by clicking the Show button. Finally, add a regression line of best fit by checking the box just to the right of the graph.
What is your best estimate for the natural solute concentration inside a potato cell? Explain how your data is evidence for that estimate.
6. Which solution is closest to being isotonic with respect to a potato cell? Which solutions were hypertonic/hypotonic? How do you know?
Challenge question:
7. Using the principles illustrated with these data, explain why you can’t drink seawater when lost at sea.
For a quick explanation of diffusion and osmosis, we highly recommend Paul Andersen’s AP Biology Lab 1: Diffusion and Osmosis video. The explanation of the potato lab starts at 5:36.
Answer key available to teachers upon request. Email [email protected]
Want an Answer Key? Fill out the form below.
Science Experiments On The Osmosis Of A Potato
Osmosis, the process in which solvent molecules move from an area of lower solute concentration to an area of higher solute concentration, can easily be demonstrated with potato experiments. Potatoes are full of both water and starch, and will gain water when immersed in watery solutions. Conversely, they will lose water when in concentrated solutions, such as those containing a great deal of starch. You can use potatoes to set up osmosis experiments for students of all ages and levels.
Potatoes in Saltwater
Cut a potato in two, and immerse one of the halves in a very salty solution of water — one containing a quarter cup of salt in a cup of water. Immerse the other piece in tap water containing no added salt. Leave both in their respective solutions for half an hour, then remove the potato halves from their solutions and observe their differences. The one in the salty solution will have shrunk, indicating that water is diffusing from a less concentrated solution to a more concentrated solution. The one in the tap water solution, in contrast, will actually swell slightly, indicating that it is taking in water.

Salt, Sugar and Pure Water
This experiment helps students to differentiate between different degrees of concentration gradients. Make one salt water solution, one sugar water solution, and for the third solution, simply use tap water. Make three thin potato slices — 1/2 cm thick. Place each potato slice into each of the solutions, and leave the slices in the solutions for a half hour.
Observe that the slice placed in salt is very flexible, while the slice placed in sugar is flexible, but less so. Since potatoes already contain sugar, less water will diffuse out of the potato placed in sugar water. The slice placed in water will be rigid, since it will absorb water.
Potato Lengths in Saline Solutions
Give your students potato "cylinders" that are uniform in length and size: for instance, you could cut them to be 70 mm in length and 7 mm in diameter. Make solutions of saline in three different concentrations, 20 percent, 0.9 percent and 0.1 percent. Have the students measure the lengths and diameters of the potato cylinders before and after soaking them in the saline solutions for half an hour. Then, have them calculate the changes in the lengths and diameters of the cylinders, and plot the saline concentrations versus the changes.
Potato Cube Weights
Cut potatoes into four groups of small, uniform cubes measuring 1/2 cm by 1/2 cm. Make four different solutions of sucrose: 10 percent, 5 percent, 1 percent and 0.01 percent. Weigh each group, on a mass balance, before immersing it in the appropriate sucrose solution for half an hour. After immersion, weigh each group again and have your students calculate the changes in the potato masses. Ask them to comment on why a group gained mass, lost mass or retained the same mass.
- The Teachers Corner: Science Experiment–Osmosis
Cite This Article
Lobo, Tricia. "Science Experiments On The Osmosis Of A Potato" sciencing.com , https://www.sciencing.com/science-experiments-osmosis-potato-8360195/. 26 April 2018.
Lobo, Tricia. (2018, April 26). Science Experiments On The Osmosis Of A Potato. sciencing.com . Retrieved from https://www.sciencing.com/science-experiments-osmosis-potato-8360195/
Lobo, Tricia. Science Experiments On The Osmosis Of A Potato last modified August 30, 2022. https://www.sciencing.com/science-experiments-osmosis-potato-8360195/
Recommended
- High School
- You don't have any recent items yet.
- You don't have any courses yet.
- You don't have any books yet.
- You don't have any Studylists yet.
- Information
Osmosis Investigation Lab
High school - us.
Students also viewed
- Biology IA Proposal
- IB Biology SL IA Final Draft
- Hadi Bajwa - 11 Photosynthesis-S
- The cell cycle
- Enzymes c1 1 ibstyle qns-1
- BIO- CLC Paper - notes and answers to help study for tests and also have all study tools
Related documents
- Analis maldicon de maliche
- Check for Understanding Meat-Poultry-Seafood
- Chapter 03 people and ideas on the move
- Czx40810 0 - The only thing I can think about
- Know Your Purpose Speech
- Ecosystems in California Estuaries - Elkhorn Slough Video and Questions
Related Studylists
Preview text, finding the osmolarity of a potato (solanum tuberosum), and a sweet potato (ipomoea batatas), december 2, 2016.
Table 1: Data showing the change in mass of potato cores when left in various concentrations of sucrose solution for 4 days. SD = standard deviation.
Table 2: Data showing the change in mass of sweet potato cores when left in various concentrations of sucrose solution for 4 days. SD = standard deviation.
Figure 1: Scatter graph showing the relationship between sucrose concentration and mean change in mass of potato cores. Error bars represent standard deviation.
that sweet potatoes have a higher osmolarity. The osmolarity of a sweet potato could be between 0 and 1 as in the solutions with this molarity there would be no change in mass (as shown through the error bars) or it could be around 0 which is where the line of best fit crosses the x-axis. However, with a potato the osmolarity is somewhere between 0 and 0 as in the solution with this molarity there would be no change in mass (as shown by the error bars). Thus concluding that sweet potato tissue has a higher osmolarity than potato tissue.
Because osmosis is defined as the passive movement of water molecules from a region of higher concentration to a region of lower concentration it is logical that sweet potato tissue has a higher osmolarity. This is due to the fact that there is more sucrose in a sweet potato than in a potato and this results in no change in mass at a solution with a higher sucrose concentration than a potato would. Similarly because the potato has less sucrose content there is no change in its mass in a solution with a lower sucrose concentration.
Looking at previous studies, I found that the osmolarity of a potato is around 0. whereas the osmolarity for the sweet potato could not be found, as there have been no past trials in this area. However, the osmolarity of a potato or sweet potato does not necessarily matter when looking at the bigger picture. It is more important to understand this concept in order for scientists to understand the osmolarity of different organs so they can be transported in the correct solutions in order for organ transplants to be successful and safe.
Evaluation:
The standard deviation was greater than zero because the values we received for each trial were quite different. For example in one trial the percentage change would be 45% and in the next it would be 12%. Thus we can conclude that there were certain things we did not hold constant, and that the experiment contained significant amounts of random errors, which explains the variation in the data collection.
There are many ways in which the method could be improved to reduce the size of the standard deviation, for example, not leaving the potato and sweet potato cores for 4 days, but instead for merely 24 hours. This would reduce their chances of becoming moldy and beginning to decompose, as a few of them did. Also, if every potato and sweet potato core had the exact same diameter and size it would make the data easier to control and might make it much more conclusive. Doing these things would therefore reduce the size of the standard deviation.
My estimates for osmolarity may be incorrect due to issues with the potatoes and sweet potatoes beginning to decompose after being left for 4 days. This resulted in the line of best fit never crossing the x-axis on the graph for potatoes. For the sweet potatoes we cannot be sure of the osmolarity due to the level of uncertainty. Since there were fluctuations in the data we cannot fully conclude that our value is the absolute osmolarity because otherwise we would have the exact same values throughout the trials.
There were some strengths to the method such as having plenty of trials for each of the different solutions and using accurate and reliable tools to measure. For each of the solutions there were 12 trials in total, and thus there is plenty of evidence we can base our claim off of. Also each group who performed the experiment used the scale and the rulers accurately to measure the potato/sweet potatoes weight and size.
In order to improve the accuracy of the results there would have to be a few minor changes to the method. These include controlling the temperature, using more concentration levels and not collecting the data in different groups. Temperature – By controlling temperature we make sure that no other outside factors affect the osmolarity of the solutions as temperature can greatly affect the rate of osmosis. For example, if the temperature increases the process of osmosis will occur faster due to the faster moving molecules, speeding up the chemical reaction overall. More Concentration Levels – If we were to use more concentrations such as 0, 0, 0, 0 (using increments of 0) we would have more results to base our conclusion and data analysis off of, and therefore have more data to create a more conclusive pattern from. This would also allow us to have more accurate results for the osmolarity of both the potato and sweet potato, as we would have more information to create a more accurate line of best fit. Collecting in Separate Groups – Because we collected our data in different groups we could not keep the way we measured constant, as everyone has different methods of measuring and rounding numbers as they measure. This would also allow each of the potato/sweet potato cores to be the same size and diameter, thus holding yet another factor constant.
Collecting more data would reduce the uncertainty because uncertainty is most commonly defined as random errors and thus solely doing more trials with more concentrations, we could reduce the level of uncertainty. However, if I were to collect more data now, I would use more precise measuring tools (such as a pipette to measure water or a thermometer to control temperature) that might show the measurement to not only two decimal places, but also three or four and to make sure everything is as precise as possible. As I stated before, I would also make sure every potato and sweet potato core had the same diameter and length to reduce the risk of having random errors due to certain variables not being held constant.
Using the results from this experiment, we can now apply this technique to other experiments. We could use it to differentiate between different species and animals through the osmolarity of their organs, or to find the difference in osmolarity between organic and inorganic vegetables.
- Multiple Choice
Subject : Biology SL

- More from: Biology SL IB (International Baccalaureate Diploma) 829 Documents Go to course
- More from: biolab by A. B. 6 6 documents Go to Studylist
Recommended for you
G11 SL IB Biology

IMAGES
COMMENTS
Clean up beakers, equipment used, table, et cetera 2 RESULTS 2.1 RESULTS AND OBSERVATION Here is the raw data table with the weight of the potatoes in pre-experiment and post-experiment forms Weight Change of Potatoes Potatoes by Pre-experimental Weight (g) Post-experimental Weight (g) Solution (mole) 0.0 20.58 21.90 0.2 17.91 18.03 0.4 23.11 ...
Experiment: Osmosis in Potatoes • Distribute two slices potato to each group. • Give each pair: – 1 Potato Activity Sheet, one 100 mL beaker of distilled water, 1 container of salt, 1 spoon, 1 petr i dish and lid labeled #1- water, 1 petri dish and lid labeled #2- salt, 2 rectangles of potato. Tell students to: 1.
In the following experiments, you will estimate the osmolarity of potato tuber cells. You will incubate pieces of potato tuber in sucrose solutions of known molarity. The object is to find the molarity at which weight of the potato tuber tissue does not change, indicating that there has been no net loss or gain of water.
Mar 26, 2020 · Pieces of potato are cut to similar sizes, weighed, and then placed in one of the six solutions overnight. The next day, the potato pieces are removed from the solutions, blotted dry, and their final masses are recorded. Each row in this tidy dataset contains an observation for a single potato piece. Each column in the dataset is a variable and ...
Apr 26, 2018 · Osmosis, the process in which solvent molecules move from an area of lower solute concentration to an area of higher solute concentration, can easily be demonstrated with potato experiments. Potatoes are full of both water and starch, and will gain water when immersed in watery solutions.
Similarly because the potato has less sucrose content there is no change in its mass in a solution with a lower sucrose concentration. Looking at previous studies, I found that the osmolarity of a potato is around 0. whereas the osmolarity for the sweet potato could not be found, as there have been no past trials in this area.
Feb 23, 2017 · Do not plagiarize this lab report, as it does not follow the IB format. Use it as a guide. Here is a model of an SL bio lab report on osmolarity. Do not plagiarize this report, but you may use it as a reference. This is a physics forum that focuses on potato osmolarity. Use it as a reference. The rough draft for the potato lab is due March 5.
This experiment in total was mostly successful and the data reported were precise and in line with what thought. Even though the way the experiment was carried out can be greatly improved in various manners. Firstly and most importantly, the equipment used to measure the masses of the potatoes could
This document describes an experiment to estimate the osmolarity of potato cells through osmosis. Potato tuber cylinders are incubated in solutions of varying sucrose concentration, then weighed to determine water gain or loss. If weight does not change, the solution and potato cells are isotonic, indicating the solution concentration matches the osmolarity of potato cells. By graphing weight ...
Dec 29, 2023 · After conducting the experiment and analyzing the data, the osmolarity of the potato core was found to be approximately 0.4 M. This value was determined by identifying the point on the graph where the percentage change in mass equaled zero, indicating isotonic conditions.