- Skip to secondary menu
- Skip to main content
- Skip to primary sidebar
Statistics By Jim
Making statistics intuitive

Retrospective Study: Definition & Examples
By Jim Frost 1 Comment
What is a Retrospective Study?
A retrospective study an experimental design that looks back in time and assesses events that have already occurred. The researchers already know the outcome for each subject when the project starts. Instead of recording data going forward as events happen, these studies use participant recollection and data that were previously recorded for reasons not relating to the project. These studies typically don’t follow patients into the future.
In retrospective designs, the researchers collect their data using existing records. Consequently, they can complete their assessment more quickly and inexpensively than a prospective study that must follow subjects over time and record the data under carefully controlled conditions. However, the data that a retrospective study uses might not have been measured consistently or accurately because they weren’t explicitly designed to be part of a study.

The statistical analysis for a retrospective study is frequently the same as for prospective designs (looking forward). The main difference is that the project occurs after the outcomes are known rather than how researchers analyze the data.
Statisticians consider retrospective designs to be inferior to prospective methods because they tend to introduce more bias and confounding. Retrospective studies are observational studies by necessity because they assess past events and it is impossible to perform a randomized, controlled experiment with them. However, they can be quicker and cheaper to complete, making them a good choice for preliminary research. Findings from a retrospective study can help inform a prospective experimental design. Learn more about Experimental Designs .
Retrospective Study Designs
Retrospective studies use various designs. While these designs differ in detail, they all tend to compare subjects with and without a condition and determine how they differ. Using the usual hypothesis tests, researchers can determine whether there are statistically significant relationships between subject variables (risk factors , personal characteristics, etc.) and the outcome of interest.
Cohort and case-control studies are standard retrospective designs. Let’s learn more about them!
Retrospective Cohort Study
This study design compares groups of subjects who are similar overall but differ in a particular characteristic, such as exposure to a risk factor. Because it is a retrospective study, the researchers find individuals where the outcomes are known when the project starts. Retrospective cohort studies frequently determine whether exposure to risk and protective factors affects an outcome. These are longitudinal studies that use existing datasets to look back at events that have already occurred. Learn more about Longitudinal Studies: Overview, Examples & Benefits .
In these projects, researchers use databases and medical records to identify patients and gather information about them. They can also ask subjects to recall their exposure over time. Then the researchers analyze the data to determine whether the risk factor correlates with the outcome of interest.
Suppose researchers hypothesize that exposure to a chemical increases skin cancer and conduct a retrospective cohort study. In that case, they can form a cohort based on a group commonly exposed to that chemical (e.g., a particular job). Then they access medical databases and records to collect their data. After identifying their subjects and obtaining the medical information, they can immediately analyze the data, comparing the outcomes for those with and without exposure.
Learn more about Cohort Studies .
Case-Control Studies
Case-control designs are generally retrospective studies. Like their cohort counterparts, case-control studies compare two groups of people, those with and without a condition. These designs both assess risk and protective factors.
Retrospective cohort and case-control studies are similar but generally have differing goals. Cohort designs typically assess known risk factors and how they affect outcomes at different times. Case-control studies evaluate a particular incident, and it is an exploratory design to identify potential risk factors.
For example, a case-control assessment might evaluate an episode of severe illness occurring after a company picnic to identify potential food culprits.
Learn more about Case-Control Studies .
Advantages of a Retrospective Study
A retrospective study tends to have the following advantages compared to a prospective design:
Cheaper : You don’t need a lab or equipment to measure information. Others did that for you!
Faster : The events have already occurred in a retrospective study—no need to wait for them to happen and then look for the differences between the groups.
Great for rare diseases : You can specifically look through a database for individuals with a rare disease or condition. In a prospective experiment, you need an immense sample size and hope enough of the rare outcomes occur for you to analyze.
Disadvantages of a Retrospective Study
Unfortunately, they tend to have the following disadvantages relating to a greater propensity for inaccuracies, inconsistencies, lack of controlled conditions, and bias:
- A retrospective study uses data measured for other purposes.
- Different people, procedures, and equipment might have recorded the data, leading to inconsistencies.
- Measurements might have occurred under differing conditions.
- Control variables might not be measured, leading to confounding.
- Recall bias.
Dean R Hess, Retrospective Studies and Chart Reviews , Respiratory Care , October 2004, 49 (10) 1171-1174.
Share this:

Reader Interactions
November 7, 2022 at 8:26 am
Coincidentally, I just read this Israeli retrospective cohort study regarding the incidence of myocarditis and pericarditis in unvaxxed post-COVID-19 patients: https://pubmed.ncbi.nlm.nih.gov/35456309/
Good news for a change.
Comments and Questions Cancel reply
Retrospective Study: Case-Control and Case-Series
Design of Experiments > Retrospective Study Contents:
- Retrospective Study
- Terminology Note
- Advantages and Disadvantages
- Study Steps
- Retrospective Case-Control
Retrospective Case Series
- Retrospective longitudinal study
1. What is a Retrospective Study?
A retrospective study is an observational study that enrolls participants who already have a disease or condition. In other words, all cases have already happened before the study begins. Researchers then look back in time, using questionnaires, medical records and other methods; Basically, you just dig into the data and see what you find. The goal is to find out what potential risk factors or other associations and relationships the group has in common. The opposite of a retrospective study is a prospective study where participants are enrolled before any of them have the disease or outcome being investigated. When both retrospective and prospective methods are used at the same time, the study is said to be ambi-directional .
Unlike most other studies, a retrospective study collects data that have been previously collected for some other reason than research (Hess, 2004).
2. Terminology Note
In epidemiology (i.e. in clinical studies), “case-control” and “retrospective study” are used synonymously. That’s mostly because when dealing with diseases and conditions, you always want to have a control. A historical epidemiological study without a control would be unthinkable, and perhaps even useless. Therefore, if you look at clinical studies, medical sites, or anything to do with medicine, you’ll find the two terms are interchangeable.
However, in other areas (e.g. education, the social sciences), there are different types of possibility for studies such as a retrospective case series , which do not use controls at all.
Back to Top
3. Advantages and Disadvantages
Advantages:
- Useful for rare diseases or unusual exposures .
- Smaller sample sizes .
- Studies take less time, because the data is readily available (it just has to be collected and analyzed).
- Costs are generally lower .
Disadvantages:
- Missing data: Exposure status may not be clear, because important data may not have been collected in the first place. For example, if the study is inverstigating occupational lung cancer rates, information about worker’s smoking habits may not be available.
- Recall bias : Participants may not be able to remember if they were exposed or not.
- Confounding variables are difficult or impossible to measure.
- Retrospective studies are considered to be inferior to prospective studies , so prospective studies should always be used if there is a choice.
- As this is a relatively weak type of study, you cannot make causal statements , although correlations are okay (see: causation vs. correlation ). Therefore, getting the study read and/or published may be difficult.
4. Study Steps
(Adpated from Kalogeropoulos, 2014):
- The study population.
- The time period (how far back in time you’ll get data from).
- Outcomes (are you studying a specific disease outcome? An event occurrence? Something else?
- Collect as much data as possible — preferably quantitative (numerical) data.
- Decide how you’ll defend your study before you implement it.
- Carefully design the database so that you’ll be able to easily analyze your results.
- Enlist other people to help, if possible. For example, you may benefit from getting a database expert to help you design your database.
5. Retrospective cohort study
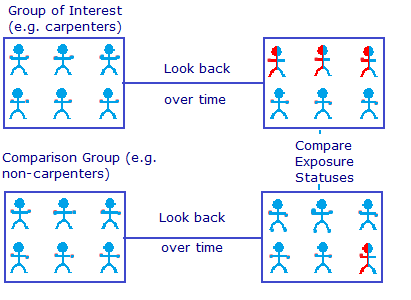
For example, researchers may want to investigate whether exposure to glues commonly used in carpentry increases the risk of developing COPD. A cohort consisting of retired carpenters might be selected. A control group is also chosen. This might be made up of delivery drivers and clerical workers who would not have been exposed to the glues. Health records and employment records are used for data sources. Due to the fact that data is collected retrospectively in a non-controlled environment, it’s not possible to make statements about causation.
Although you can’t make statements about causation , you can find associations and possible relationships, potentially paving the way for the more expensive, longer-term prospective study .
6. Types of Retrospective cohort study
Retrospective case-control study.
Case-control studies involve two groups of people: people who have the disease (cases) and those who do not (controls). A retrospective case-control uses these two groups and looks back to the past for data and possible risk factors. A matched case-control study chooses controls based on some matching factor, like age, weight or severity of disease.
7. Retrospective longitudinal study
A retrospective longitudinal study involves repeated observations of the variables over a long period of time.
Hess, D. (2004). Retrospective studies and chart reviews. Respiratory Care. 2004 Oct;49(10):1171-4. Kalogeropoulos, A. (2014). Understanding Retrospective vs. Prospective Study designs. Retrieved October 26, 2017 from: http://medicine.emory.edu/documents/research/kalogeropoulos-study-design-talk.pdf Sahai, H. & Khurshid, A. (1995). Statistics in Epidemiology: Methods, Techniques and Applications . CRC Press.
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
Methodology
- What Is a Retrospective Cohort Study? | Definition & Examples
What Is a Retrospective Cohort Study? | Definition & Examples
Published on February 10, 2023 by Tegan George . Revised on June 22, 2023.
A retrospective cohort study is a type of observational study that focuses on individuals who have an exposure to a disease or risk factor in common. Retrospective cohort studies analyze the health outcomes over a period of time to form connections and assess the risk of a given outcome associated with a given exposure.
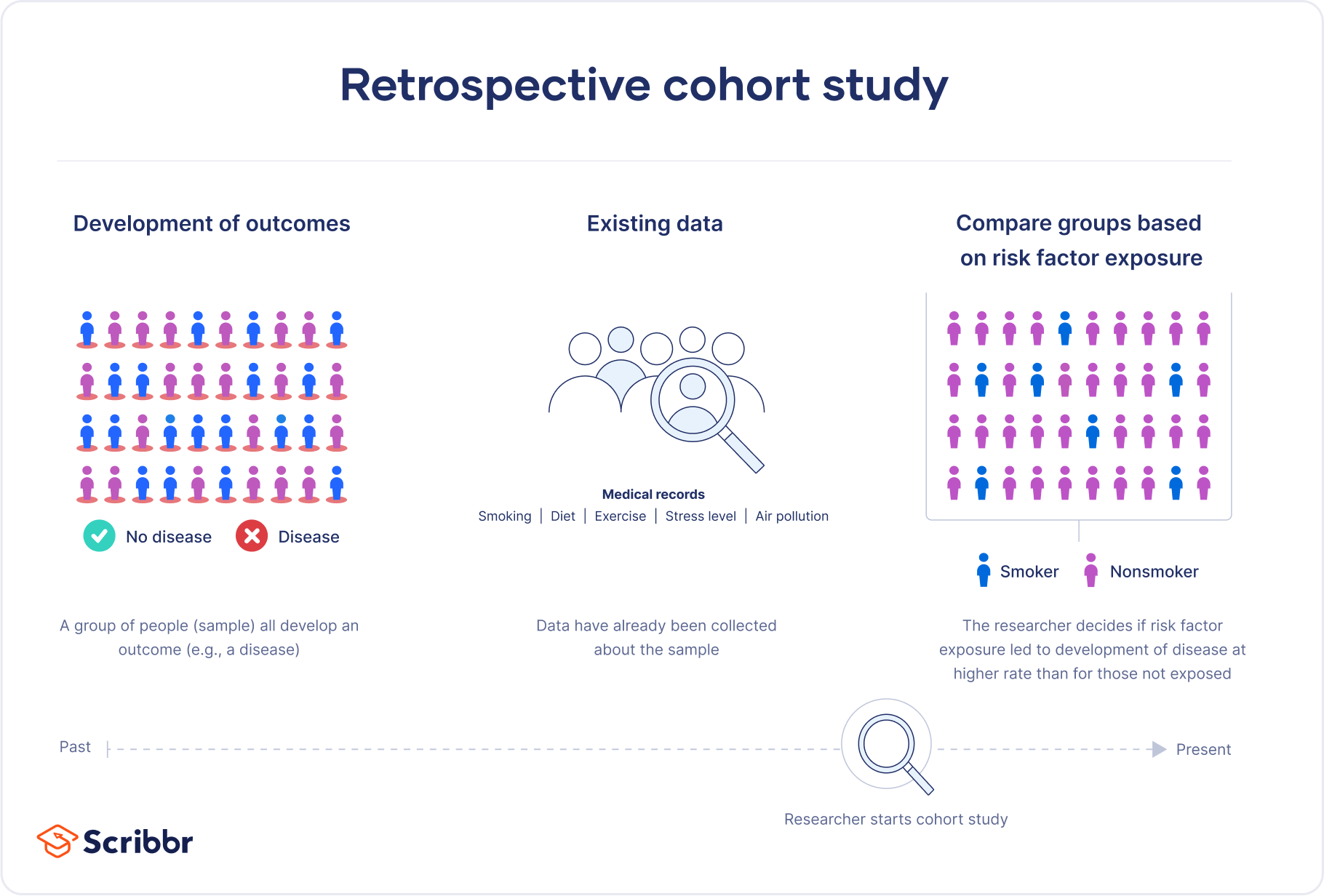
It is crucial to note that in order to be considered a retrospective cohort study, your participants must already possess the disease or health outcome being studied.
Table of contents
When to use a retrospective cohort study, examples of retrospective cohort studies, advantages and disadvantages of retrospective cohort studies, other interesting articles, frequently asked questions.
Retrospective cohort studies are a type of observational study . They are often used in fields related to medicine to study the effect of exposures on health outcomes. While most observational studies are qualitative in nature, retrospective cohort studies are often quantitative , as they use preexisting secondary research data. They can be used to conduct both exploratory research and explanatory research .
Retrospective cohort studies are often used as an intermediate step between a weaker preliminary study and a prospective cohort study , as the results gleaned from a retrospective cohort study strengthen assumptions behind a future prospective cohort study.
A retrospective cohort study could be a good fit for your research if:
- A prospective cohort study is not (yet) feasible for the variables you are investigating.
- You need to quickly examine the effect of an exposure, outbreak, or treatment on an outcome.
- You are seeking to investigate an early-stage or potential association between your variables of interest.
Retrospective cohort studies use secondary research data, such as existing medical records or databases, to identify a group of people with an exposure or risk factor in common. They then look back in time to observe how the health outcomes developed. Case-control studies rely on primary research , comparing a group of participants with a condition of interest to a group lacking that condition in real time.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Retrospective cohort studies are common in fields like medicine, epidemiology, and healthcare.
You collect data from participants’ exposure to organophosphates, focusing on variables like the timing and duration of exposure, and analyze the health effects of the exposure. Example: Healthcare retrospective cohort study You are examining the relationship between tanning bed use and the incidence of skin cancer diagnoses.
Retrospective cohort studies can be a good fit for many research projects, but they have their share of advantages and disadvantages.
Advantages of retrospective cohort studies
- Retrospective cohort studies are a great choice if you have any ethical considerations or concerns about your participants that prevent you from pursuing a traditional experimental design .
- Retrospective cohort studies are quite efficient in terms of time and budget. They require fewer subjects than other research methods and use preexisting secondary research data to analyze them.
- Retrospective cohort studies are particularly useful when studying rare or unusual exposures, as well as diseases with a long latency or incubation period where prospective cohort studies cannot yet form conclusions.
Disadvantages of retrospective cohort studies
- Like many observational studies, retrospective cohort studies are at high risk for many research biases . They are particularly at risk for recall bias and observer bias due to their reliance on memory and self-reported data.
- Retrospective cohort studies are not a particularly strong standalone method, as they can never establish causality . This leads to low internal validity and external validity .
- As most patients will have had a range of healthcare professionals involved in their care over their lifetime, there is significant variability in the measurement of risk factors and outcomes. This leads to issues with reliability and credibility of data collected.
If you want to know more about statistics , methodology , or research bias , make sure to check out some of our other articles with explanations and examples.
- Student’s t -distribution
- Normal distribution
- Null and Alternative Hypotheses
- Chi square tests
- Confidence interval
- Quartiles & Quantiles
- Cluster sampling
- Stratified sampling
- Data cleansing
- Reproducibility vs Replicability
- Peer review
- Prospective cohort study
Research bias
- Implicit bias
- Cognitive bias
- Placebo effect
- Hawthorne effect
- Hindsight bias
- Affect heuristic
- Social desirability bias
The primary difference between a retrospective cohort study and a prospective cohort study is the timing of the data collection and the direction of the study.
A retrospective cohort study looks back in time. It uses preexisting secondary research data to examine the relationship between an exposure and an outcome. Data is collected after the outcome you’re studying has already occurred.
Alternatively, a prospective cohort study follows a group of individuals over time. It collects data on both the exposure and the outcome of interest as they are occurring. Data is collected before the outcome of interest has occurred.
Retrospective cohort studies are at high risk for research biases like recall bias . Whenever individuals are asked to recall past events or exposures, recall bias can occur. This is because individuals with a certain disease or health outcome of interest are more likely to remember and/or report past exposures differently to individuals without that outcome. This can result in an overestimation or underestimation of the true relationship between variables and affect your research.
No, retrospective cohort studies cannot establish causality on their own.
Like other types of observational studies , retrospective cohort studies can suggest associations between an exposure and a health outcome. They cannot prove without a doubt, however, that the exposure studied causes the health outcome.
In particular, retrospective cohort studies suffer from challenges arising from the timing of data collection , research biases like recall bias , and how variables are selected. These lead to low internal validity and the inability to determine causality.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
George, T. (2023, June 22). What Is a Retrospective Cohort Study? | Definition & Examples. Scribbr. Retrieved October 8, 2024, from https://www.scribbr.com/methodology/retrospective-cohort-study/
Is this article helpful?
Tegan George
Other students also liked, what is a case-control study | definition & examples, what is an observational study | guide & examples, what is recall bias | definition & examples, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts

LEARN STATISTICS EASILY
Learn Data Analysis Now!

What is: Retrospective Study
What is a retrospective study.
A retrospective study is a research design that examines data from past events to identify patterns, correlations, or outcomes. This type of study is often used in fields such as epidemiology, social sciences, and clinical research. By analyzing existing data, researchers can gain insights into the relationships between variables without the need for a prospective data collection process.
Ad description. Lorem ipsum dolor sit amet, consectetur adipiscing elit.

Characteristics of Retrospective Studies
Retrospective studies typically involve the analysis of historical data, which can include medical records, survey responses, or previously collected datasets. One of the key characteristics of this study design is that it looks backward in time, often focusing on events that have already occurred. This allows researchers to identify associations and potential causal relationships between variables, although it does not establish causation definitively.
Types of Retrospective Studies
There are several types of retrospective studies, including case-control studies and cohort studies. In a case-control study, researchers compare individuals with a specific outcome (cases) to those without it (controls) to identify potential risk factors. In contrast, cohort studies involve following a group of individuals over time, looking back at their exposure to certain variables to determine how these exposures may have influenced outcomes.
Advantages of Retrospective Studies
One of the primary advantages of retrospective studies is their cost-effectiveness and efficiency. Since the data has already been collected, researchers can save time and resources compared to prospective studies. Additionally, retrospective studies can provide valuable insights into rare diseases or outcomes, as they allow researchers to analyze a larger pool of historical data that may not be feasible to collect in real-time.
Limitations of Retrospective Studies
Despite their advantages, retrospective studies have limitations. One significant concern is the potential for bias, particularly selection bias and recall bias. Selection bias occurs when the individuals included in the study are not representative of the general population, while recall bias arises when participants do not accurately remember past events. These biases can affect the validity of the study’s findings and limit the ability to draw definitive conclusions.
Data Sources for Retrospective Studies
Researchers conducting retrospective studies often rely on various data sources, including electronic health records, administrative databases, and historical survey data. These sources can provide a wealth of information, but researchers must ensure the data’s quality and completeness. Proper data management and cleaning are essential to minimize errors and biases in the analysis.
Statistical Analysis in Retrospective Studies
Statistical analysis plays a crucial role in retrospective studies, as it helps researchers identify significant associations and control for confounding variables. Common statistical methods used include logistic regression, chi-square tests, and survival analysis. These techniques allow researchers to interpret the data effectively and draw meaningful conclusions from their findings.
Ethical Considerations
Ethical considerations are paramount in retrospective studies, particularly when dealing with sensitive data such as medical records. Researchers must ensure that they have the appropriate permissions to access and use the data, and they must adhere to ethical guidelines to protect the privacy and confidentiality of participants. Institutional Review Board (IRB) approval may be required, depending on the nature of the study.
Applications of Retrospective Studies
Retrospective studies are widely used in various fields, including public health, epidemiology, and social sciences. They can help identify risk factors for diseases, evaluate treatment outcomes, and inform public health policies. By leveraging existing data, researchers can contribute to the body of knowledge in their respective fields and guide future research directions.

Cohort Studies
- 1
- | 2
- | 3
- | 4
- | 5
- | 6

Prospective Versus Retrospective Cohort Studies
Prospective cohort studies:, retrospective cohort studies, an ambidirectional cohort study, closed (fixed) versus open cohorts.

Epi_Tools.XLSX
All Modules
There are two fundamental types of cohort studies based on when and how the subjects are enrolled into the study:
In prospective cohort studies the investigators conceive and design the study, recruit subjects, and collect baseline exposure data on all subjects, before any of the subjects have developed any of the outcomes of interest. The subjects are then followed into the future in order to record the development of any of the outcomes of interest. The follow up can be conducted by mail questionnaires, by phone interviews, via the Internet, or in person with interviews, physical examinations, and laboratory or imaging tests. Combinations of these methods can also be used.

Typically, the investigators have a primary focus, for example, to learn more about cardiovascular disease or cancer, but the data collected from the cohort over time can be used to answer many questions and test many possible determinants, even factors that they hadn't considered when the study was originally conceived.
The Framingham Heart Study , the Nurses Health Study , and the Black Women's Health Study are good examples of large, productive prospective cohort studies. In each of these studies, the investigators wanted to study risk factors for common chronic diseases. The investigators identified a cohort (group) of possible subjects who would be feasible to follow for a prolonged period. Eligible subjects had to meet certain criteria (inclusion criteria) to be included in the study as subjects. The investigators then determine the initial or "baseline" characteristics, behaviors, and other "exposures" of all subjects at the beginning of the study. Information is collected from all subjects in the same way using exactly the same questions and data collection methods for all subjects. They design the questions and data collection procedures very carefully in order to have accurate information about exposures before disease develops in any of the subjects.
For more information:
Link to Framingham Heart Study
Link to The Nurses Health Study
Link to The Black Women's Health Study
Of course, data analysis cannot take place until enough 'events' or 'outcomes' have occurred, so time must elapse, and the analyses will look at events that have occurred during the period of time from the beginning of the study until the time of the analysis or the end of the study. It goes without saying that analysis is always done retrospectively, because a span of time has to have elapsed before you can compare incidence. The thing that makes prospective cohort studies prospective is that they were designed prospectively, and subjects were enrolled and had baseline data collected before any of them developed any of the outcomes of interest. Determining baseline exposure status before disease events occur gives prospective studies an important advantage in reducing certain types of bias that can occur in retrospective cohort studies and case-control studies, though at the cost of efficiency.
The illustration below shows a hypothetical group of 12 subjects followed over a number of years. They were enrolled into the study at different times, and some of them became lost to follow up, i.e., they stopped responding to letters, emails and phone calls, so we don't know what happened to them; these are show by the horizontal follow up line stopping.

Three subjects developed the outcome of interest at the approximate dates show by the "X"s. The incidence rate was calculated by computing the disease free observation time for each subject, adding up the disease-free observation times for the entire group, and then dividing this into the number of events, as shown in the calculation below the time line.
Since the investigators asked about many exposures during baseline data collection, they can eventually use the data to study many associations between different exposures and disease outcomes. For example, one could identify smokers and non-smokers at baseline and compare their subsequent incidence of developing heart disease. Alternatively, one could group subjects based on their body mass index (BMI) and compare their risk of developing heart disease or cancer.
| Body Mass Index | # Non-fatal Heart Attacks | Person-Years of Observation | MI Rate per 100,000 Person-Years | Rate Ratio |
| <21 | 41 | 177,356 | 23.1 | 1.0 |
| 21-23 | 57 | 194,243 | 29.3 | 1.3 |
| 23-25 | 56 | 155,717 | 36.0 | 1.6 |
| 25-29 | 67 | 148,541 | 45.1 | 2.0 |
| >29 | 85 | 99,573 | 85.4 | 3.7 |
There were over 118,000 nurses in the study, and they divided the cohort int0 five exposure groups based on BMI. In this case they used the incidence rate of myocardial infarctions (MI, i.e., heart attacks) in the leanest women (BMI < 21) as a reference , against which they compared the incidence rates of MI in the other four groups. For example, the incidence rate of MI in the reference group (those with BMI < 21) was 23.1 per 100,000 person-years of disease-free observation time. The incidence rate in the heaviest group (BMI > 29) was 85.4 MIs per 100,000 person-years.
The Epi-Tools.XLS worksheet for cohort studies can compare either cumulative incidence (top section of the worksheet) or incidence rates like these (lower section of the worksheet). For example, if one were to compare the heaviest group (BMI > 29) to the women with BMI < 21 (the reference group), the Epi-Tools analysis would look like this

Link to the article by Manson et al.
Retrospective studies also group subjects based on their exposure status and compare their incidence of disease. However, in this case both exposure status and outcome are ascertained retrospectively.

Retrospective cohort studies are particularly useful for unusual exposures or occupational exposures. For example, if an investigator wanted to determine whether exposure to chemicals used in tire manufacturing was associated with an increased risk of death, one might find a tire manufacturing factory that had been in operation for several decades. One could potentially use employee health records to identify those who had had jobs which involved exposure to the chemicals in question (e.g., workers who actually manufactured tires) and non-exposed coworkers (e.g., clerical workers or sales personnel in the same company or, even better, workers also involved in manufacturing operations but with jobs that didn't involve exposure to the chemicals). One could then ascertain what had happened to all the subjects and compare the incidence of death in the exposed and non-exposed workers.
Retrospective cohort studies like this are very efficient because they take much less time and cost much less than prospective cohort studies, but this advantage also creates potential problems. Sometimes exposure status is not clear when it is necessary to go back in time and use whatever data was available, because the data being used was not designed to be used in a study. Even if it was clear who was exposed to tire manufacturing chemicals based on employee records, it would also be important to take into account (or adjust for) other differences that could have influenced mortality (confounding factors). For example, in a study comparing mortality rates between workers exposed to solvents used in tire manufacture and an unexposed comparison group, it might be important to adjust for confounding factors such as smoking and alcohol consumption. However, it is unlikely that a retrospective cohort study would have accurate information on these other risk factors.

A cohort study may also be ambidirectional , meaning that there are both retrospective and prospective phases of the study. Ambidirectional studies are much less common than purely prospective or retrospective studies, but they are conceptually consistent with and share elements of the advantages and disadvantages of both types of studies. The Air Force Health Study (AFHS) - also known as the Ranch Hand Study - was initiated by the U. S. Air Force in 1979 to assess the possible health effects of military personnel's exposure to Agent Orange and other chemical defoliants sprayed during the Vietnam War. The study was conducted comparing:
- 1,098 pilots exposed to dioxin in Vietnam (Operation Ranch Hand)
- 1,549 men who flew cargo missions in Southeast Asia during the same time

This is an "ambidirectional" study, because it had both a retrospective component and a prospective component. Some of the problems suspected to be caused by Agent Orange would have occurred shortly after exposure (e.g., skin rashes). These were addressed by looking at the cohort retrospectively to see if the exposed pilots had had more problems than the controls. Other problems (e.g., infertility & cancer) might not surface until some time after the exposure. Therefore, the cohort was followed prospectively to see if they had a greater incidence of these problems. The reports that emerged from the study suggested links between Agent Orange exposure and nine distinct diseases: chloracne, Hodgkin's disease, multiple myeloma, non- Hodgkin's lymphoma, porphyria cutanea tarda, respiratory cancers (lung, bronchus, larynx and trachea), soft-tissue sarcoma, acute and subacute peripheral neuropathy, and prostate cancer.
A closed cohort is one with fixed membership. Once the cohort is defined by enrolling subjects and follow up begins, no one can be added. The number of subjects may decline because of death or loss to follow up, but no additional subjects are added. As a result, closed cohorts always get smaller over time. Citizens of Japan who were exposed to radiation when atomic bombs were dropped on Hiroshima and Nagasaki during the second World War, would be considered members of a fixed or closed cohort that was defined by an event. Ashengrau and Seage would classify the bombing victims as a "fixed cohort" and make a distinction between a fixed cohort and a closed cohort. They define a closed cohort as similar to a fixed cohort except that a closed cohort is one that has no losses to follow up, for example, a cohort of people who attended a luncheon that resulted in an outbreak of Salmonellosis.
In contrast, an open cohort is dynamic, meaning that members can leave or be added over time. Rothman gives the example of a state cancer registry. Subjects are continually added when they are diagnosed with cancer, so new subjects are continually added. Subjects can also leave the cohort by moving to a new state or dying. Another example of an open or dynamic cohort would be students at Boston University.
These descriptions should sound familiar, because they essentially parallel the descriptions of fixed and dynamic populations from the Measures of Disease Frequency module. The great majority of cohort studies are conducted in closed (or fixed) cohorts, because it is more difficult to establish eligibility and track people in an open cohort, since they can enter and leave at any time. This problem becomes greater as the size of the cohort gets larger and/or the study continues for a longer period of time. Note that the retrospective cohort study of Giardia in Milton was an open cohort (members of the golf club), but the population was relatively small and time period very short.
return to top | previous page | next page
Content ©2016. All Rights Reserved. Date last modified: September 21, 2016. Wayne W. LaMorte, MD, PhD, MPH
- Skip to main content
- Skip to primary sidebar
- Skip to footer
- QuestionPro

- Solutions Industries Gaming Automotive Sports and events Education Government Travel & Hospitality Financial Services Healthcare Cannabis Technology Use Case AskWhy Communities Audience Contactless surveys Mobile LivePolls Member Experience GDPR Positive People Science 360 Feedback Surveys
- Resources Blog eBooks Survey Templates Case Studies Training Help center
Home Surveys Academic Research
Retrospective Study: What it is & How to Do it

In a retrospective study , existing data are examined to identify risk factors for particular diseases. Interpretations are limited as it is impossible to go back in time and gather the missing information. Let’s talk about that.
What is a Retrospective Study?
A retrospective study is an analysis that compares two groups of individuals. A case group and a control group. Both groups are similar, but the case group has a key factor that is being studied or investigated that the control group does not.
It brings up key differences in individuals or groups of individuals based on some events/incidences. It’s a psychological approach to pinpoint key differences in individuals that are alike but differ slightly based on certain characteristics.
What is the importance of a Retrospective Study?
This study is greatly important in many aspects, be it personal development, socio-economic welfare, or professional development.
This study is also important to fixate certain characteristics within individuals by considering historical data and making rational decisions. In large organizations, this becomes very important because organizations deal with large groups of clients with almost (but not completely) alike behaviors.
This is the stage where retrospection comes into play to understand differences and similarities among clients and act accordingly.
Advantages and disadvantages of a Retrospective Study
Similar to other studies, there are benefits and drawbacks to retrospective studies. Researchers must critically evaluate the method that is used and carefully interpret the results of retrospective studies before putting them to practice.
Advantages:
- Researches have control of the number of participants in the case group and control group.
- Typically less expensive to conduct in comparison to other methods.
- Timeline of a retrospective study is quicker to complete
Disadvantages:
- Poor control over exposure factor
- If research is not thorough, there could be missing history/background and or information.
- If researchers are not careful, selection and recall bias can affect the results
How to conduct a Retrospective Study
There are certain ways to conduct a retrospective study. Most of them collect vast historical data and make sense of it statistically. Another way is by considering 2 groups with similar attributes and then conducting Surveys (That’s where QuestionPro shines!) to find out differing characteristics. In general, there are 2 parts to it:
Prospective Study
Retrospective study.
For the sake of this article, let’s just stick to the retrospective study. An organization can take many surveys and collect data from them. This data can then be processed to make important business decisions. This will help the organization understand its clients and help provide more viable products and services that would actually make a difference.
Retrospective Study Examples
These studies aim to study a situation, condition, event, or phenomenon that has already happened. This study is often used in the medical industry to study different medical conditions and illnesses. Using participants who have an existing condition compared to a group that does not have that particular condition.
Conclusion:
In conclusion, this study deals with enhancing minor differences amongst similar groups. These differences are brought up by studying and statistically sourcing historical data collected by various means. One such method to collect data and perform this study is by taking surveys.
QuestionPro has a rich arsenal of tools and products to bring the most out of such studies. These tools collect useful insights and help in procuring pinpoint differences within the most similar groups of individuals and further accelerating the study.
CREATE FREE ACCOUNT
Authors : Siddharth Kulkarni & Jenny Huang
MORE LIKE THIS

Pulse Surveys vs Annual Employee Surveys: Which to Use
Oct 4, 2024

Employee Perception Role in Organizational Change
Oct 3, 2024

Mixed Methods Research: Overview of Designs and Techniques
Oct 2, 2024

Customer Engagement Platforms to Boost Interaction
Oct 1, 2024
Other categories
- Academic Research
- Artificial Intelligence
- Assessments
- Brand Awareness
- Case Studies
- Communities
- Consumer Insights
- Customer effort score
- Customer Engagement
- Customer Experience
- Customer Loyalty
- Customer Research
- Customer Satisfaction
- Employee Benefits
- Employee Engagement
- Employee Retention
- Friday Five
- General Data Protection Regulation
- Insights Hub
- Life@QuestionPro
- Market Research
- Mobile diaries
- Mobile Surveys
- New Features
- Online Communities
- Question Types
- Questionnaire
- QuestionPro Products
- Release Notes
- Research Tools and Apps
- Revenue at Risk
- Survey Templates
- Training Tips
- Tuesday CX Thoughts (TCXT)
- Uncategorized
- What’s Coming Up
- Workforce Intelligence

Retrospective Studies and Chart Reviews
Chris nickson.
- Nov 3, 2020
- Retrospective studies are designed to analyse pre-existing data, and are subject to numerous biases as a result
- Retrospective studies may be based on chart reviews (data collection from the medical records of patients)
- case series
- retrospective cohort studies (current or historical cohorts)
- case-control studies
STATISTICAL ANALYSIS USED IN RETROSPECTIVE STUDIES
- Compare outcomes between treatment and control group
- Used if treatment and control group are selected by a chance mechanism
- Divide all patients into subgroups according to a risk factor, then perform comparison within these subgroups
- Used if only one key confounding variable exists
- Find pairs of patients that have specific characteristics in common, but received different treatments; compares outcome only in these pairs
- Used if only a few confounders exist and if the size of one of the comparison groups is much larger than the other
- More than one confounder is controlled simultaneously, if a larger number of confounders needs to be adjusted for computer software and statistical advice is necessary
- Used if sample size is large
- Simple description of data
- Used if sample size is low and other options failed
ADVANTAGES OF RETROSPECTIVE STUDIES
- quicker, cheaper and easier than prospective cohort studies
- can address rare diseases and identify potential risk factors (e.g. case-control studies)
- not prone to loss of follow up
- may be used as the initial study generating hypotheses to be studied further by larger, more expensive prospective studies
DISADVANTAGES OF RETROSPECTIVE STUDIES
- inferior level of evidence compared with prospective studies
- controls are often recruited by convenience sampling, and are thus not representative of the general population and prone to selection bias
- prone to recall bias or misclassification bias
- subject to confounding (other risk factors may be present that were not measured)
- cannot determine causation, only association
- some key statistics cannot be measured
- temporal relationships are often difficult to assess
- retrospective cohort studies need large sample sizes if outcomes are rare
SOURCES OF ERROR IN CHART REVIEWS AND THEIR SOLUTIONS
From Kaji et al (2014) and Gilbert et al (1996):
- establish whether necessary information is available in the charts
- establish if there are sufficient charts to perform the analysis with adequate precision
- perform a sample size calculation
- Declare any conflict of interest Provide evidence of institutional review board approval
- Submit the data collection form, as well as the coding rules and definitions, as an online appendix
- Case selection or exclusion using explicit protocols and well described the criteria
- Ensure all available charts have an equal chance of selection
- Provide a flow diagram showing how the study sample was derive from the source population
- define the predictor and outcome variables to be collected a priori
- Develop a coding manual and publish as an online appendix
- Use standardized abstraction forms to guide data collection
- Provide precise definitions of variables
- Pilot test the abstraction form
- Ensure uniform handling of data that is conflicting, ambiguous, missing, or unknown
- Perform a sensitivity analysis if needed
- Blind chart reviewers to the etiologic relation being studied or the hypotheses being tested. If groups of patients are to be compared, the abstractor should be blinded to the patient’s group assignment
- Describe how blinding was maintained in the article
- Train chart abstractors to perform their jobs.
- Describe the qualifications and training of the chart abstracters.
- Ideally, train abstractors before the study starts, using a set of “practice” medical records.
- Ensure uniform training, especially in multi-center studies
- Monitor the performance of the chart abstractors
- Hold periodic meetings with chart abstractors and study coordinators to resolve disputes and review coding rules.
- A second reviewer should re-abstract a sample of charts, blinded to the information obtained by the first correlation reviewer.
- Report a kappa-statistic, intraclass coefficient, or other measure of agreement to assess inter-rater reliability of the data
- Provide justification for the criteria for each variable
SOURCES OF ERROR FROM THE USE OF ELECTRONIC MEDICAL RECORDS
Potential biases introduced from:
- use of boilerplates (a unit of writing that can be reused over and over without change)
- items copied and pasted
- default tick boxes
- delays in time stamps relative to actual care
References and Links
- CCC — Case-control studies
Journal articles
- Gilbert EH, Lowenstein SR, Koziol-McLain J, Barta DC, Steiner J. Chart reviews in emergency medicine research: Where are the methods? Ann Emerg Med. 1996 Mar;27(3):305-8. PMID: 8599488 .
- Kaji AH, Schriger D, Green S. Looking through the retrospectoscope: reducing bias in emergency medicine chart review studies. Ann Emerg Med. 2014 Sep;64(3):292-8. PMID: 24746846 .
- Sauerland S, Lefering R, Neugebauer EA. Retrospective clinical studies in surgery: potentials and pitfalls. J Hand Surg Br. 2002 Apr;27(2):117-21. PMID: 12027483 .
- Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005 Apr;45(4):448-51. PMID: 15795729 .

Critical Care
Chris is an Intensivist and ECMO specialist at the Alfred ICU in Melbourne. He is also a Clinical Adjunct Associate Professor at Monash University . He is a co-founder of the Australia and New Zealand Clinician Educator Network (ANZCEN) and is the Lead for the ANZCEN Clinician Educator Incubator programme. He is on the Board of Directors for the Intensive Care Foundation and is a First Part Examiner for the College of Intensive Care Medicine . He is an internationally recognised Clinician Educator with a passion for helping clinicians learn and for improving the clinical performance of individuals and collectives.
After finishing his medical degree at the University of Auckland, he continued post-graduate training in New Zealand as well as Australia’s Northern Territory, Perth and Melbourne. He has completed fellowship training in both intensive care medicine and emergency medicine, as well as post-graduate training in biochemistry, clinical toxicology, clinical epidemiology, and health professional education.
He is actively involved in in using translational simulation to improve patient care and the design of processes and systems at Alfred Health. He coordinates the Alfred ICU’s education and simulation programmes and runs the unit’s education website, INTENSIVE . He created the ‘Critically Ill Airway’ course and teaches on numerous courses around the world. He is one of the founders of the FOAM movement (Free Open-Access Medical education) and is co-creator of litfl.com , the RAGE podcast , the Resuscitology course, and the SMACC conference.
His one great achievement is being the father of three amazing children.
On Twitter, he is @precordialthump .
| INTENSIVE | RAGE | Resuscitology | SMACC
Leave a Reply Cancel reply
This site uses Akismet to reduce spam. Learn how your comment data is processed .

IMAGES
VIDEO
COMMENTS
What is a Retrospective Study? A retrospective study an experimental design that looks back in time and assesses events that have already occurred. The researchers already know the outcome for each subject when the project starts.
1. What is a Retrospective Study? A retrospective study is an observational study that enrolls participants who already have a disease or condition. In other words, all cases have already happened before the study begins.
A retrospective cohort study is a type of observational study focused on individuals who have an exposure to a disease or risk factor in common.
A retrospective study is a research design that examines data from past events to identify patterns, correlations, or outcomes. This type of study is often used in fields such as epidemiology, social sciences, and clinical research.
Retrospective cohort studies are also 'longitudinal,' because they examine health outcomes over a span of time. The distinction is that in retrospective cohort studies all of the cases of disease have already occurred before the investigators initiate the study.
A retrospective cohort study, also known as a historical cohort study, is a type of observational study where the researcher looks back in time at historical data to examine the relationship between certain risk factors or exposures and outcomes.
A retrospective study is an analysis that compares two groups of individuals. A case group and a control group. Both groups are similar, but the case group has a key factor that is being studied or investigated that the control group does not. It brings up key differences in individuals or groups of individuals based on some events/incidences.
A retrospective study (by definition non-interventional) is a purely observational review and/or a reassessment of database records to analyze events of interest that have already happened.
Retrospective studies are designed to analyse pre-existing data, and are subject to numerous biases as a result. Retrospective studies may be based on chart reviews (data collection from the medical records of patients) Types of retrospective studies include: case series. retrospective cohort studies (current or historical cohorts)
The term retrospective study describes case–control studies and other designs, such as historical cohort studies, in which relevant exposures and/or disease incidences occurred before the time of the study data collection. Citing Literature.