Get Your ALL ACCESS Shop Pass here →


Solid Liquid Gas Experiment
Looking to set up a quick and simple changing states of matter experiment with kids? This solid, liquid, and gas activity uses very few supplies but demonstrates the concepts so easily! Plus, make sure to grab the free printable States of Matter mini pack.

Solid, Liquid, and Gas Experiment
Explore the 3 states of matter with ice, water and steam!
💡 Here are more fun states of matter science experiments to explore!
Watch the Video:
- large bowl or two
- tongs (optional)
Experiments Set Up
Step 1: Fill a bowl full of ice! Here’s the solid-frozen water.

Step 2: Let the ice melt! Here’s the liquid – water.
Quick Tip: Save time by adding warm water to the bowl or bring out a bowl of water to use. We talked about how water is still matter, but it flows and has a shape that changes.

Step 3: Adults only! Carefully boil the water. The steam is the gas!

Optional, if safe to do so, allow your kid to feel the steam. What does it feel like?

States of Matter For Kids
What is matter? In science, matter refers to any substance that has mass and takes up space. Matter consists of tiny particles called atoms , and it has different forms depending on how the atoms are arranged. This is what we call states of matter .
What are three states of matter?
The three states of matter are solid (ice), liquid (water), and gas (steam). Although a fourth state of matter exists, called plasma, it’s not shown in any demonstrations.
💡 Our baking soda and vinegar balloon experiment also shows 3 states of matter!
What are the differences between the states of matter?
Solid: A solid has tightly packed particles in a specific pattern, which cannot move about. You will notice a solid keeps its own shape. Ice or frozen water keeps its shape.
Liquid: In a liquid, the particles have some space between them with no pattern, so they are not in a fixed position. A liquid has no distinct shape but will take the shape of a container it is put into. Water can be poured and takes the shape of the container it is in.
Gas: In a gas, the particles move freely from one another. You can also say they vibrate! Gas particles spread out to take the shape of the container they are put in. Steam or water vapor is an example of a gas.
Try This Free States of Matter Activity!

Explore More States of Matter Experiments
Check out all our states of matter experiments for kids , and also learn about phase changes .
💡 For preschool and kindergarten these ice play activities are a playful and hands-on way to introduce states of matter.
- Melting Chocolate: Give each child a piece of chocolate and let them hold it in their hands to see it melt. Compare with chocolate in a cool spot.
- Oobleck: Mix cornstarch with water to create a substance that acts like both a solid and a liquid.
- Baking Soda & Vinegar Balloon: Observe how adding a solid to a liquid produces a gas that inflates the balloon.
- Frost on a Can: Fill a metal can with ice and observe water droplets forming on the outside. Observe how gases (water vapor) in the air turn into liquid when cooled.
- Bubble Fun: Use bubble wands to blow bubbles and observe how liquids can trap gas to form bubbles.
- Melting Ice: Explore different ways to make ice melt faster.
- Hot Chocolate : Explore states of matter with a tasty drink and free printable!

Helpful Science Resources
Here are a few resources that will help you introduce science more effectively to your kids or students and feel confident presenting materials. You’ll find helpful free printables throughout.
- Best Science Practices (as it relates to the scientific method)
- Science Vocabulary
- All About Scientists
- Free Science Worksheets
- DIY Science Kits
- Science Tools for Kids
- Scientific Method for Kids
- Citizen Science Guide
- Join us in the Club
- Printable Science Project Pack

Awesome! Looks like Liam enjoyed this.
Sarah, I love this post! Such a great idea and I could jump in my kitchen and do it right now! Thank you for continuing to inspire our learning at home – Liam is such a lucky little man!
This looks fun! I always considered matter are too complicated (for preschooler and me!) but this is explained in a child-friendly way. (Even I understand it! lol!) I’m totally going to have to look for that Bernstein Bears book!
Very simple way to show the states of matter. Thanks for sharing at Mom’s Library!
Featured you this week!
- Pingback: Liquid Density Tower Physics Activity and Experiment for Kids
- Pingback: Frozen Dinosaur Eggs Ice Melt Science Activity | Little Bins for Little Hands
- Pingback: 15 States of Matter Science Experiments for Kids - Mom For All Seasons
Great idea and presentation,I tweaked it just a little and poured warm water over the ice to make steam and then of course it is the liquid! Thanks for sharing!
Comments are closed.

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.


- Separation Techniques
- Qualitative Analysis
- Kinetic Theory
- Atomic Structure
- Stoichiometry
- Acids, Bases & Salts
- Energy from Chemicals
- Speed of Reaction
- Electrolysis
- The Periodic Table
- N Level Questions
- O Level Combined Questions
- O Level Pure Questions
- Data Based Questions
- Coffee with Chemists
Gas collection: water displacement, upward delivery & downward delivery
The three methods of collecting gases by their physical properties

1. Water displacement: trap in water
For gases that do not dissolve in water, we can trap them in water using a method with the fancy name of displacement of water .
This method requires a delivery tube , a measuring cylinder , and a basin of water .
We first fill a measuring cylinder with water and invert it in a basin of water. We then use a delivery tube to bubble gas into the inverted measuring cylinder. As gas bubbles and fills up the inverted measuring cylinder, the water level drops.
2. Upward delivery: let it float
Air is a mixture of mainly nitrogen and oxygen gas.
Gases that are less dense than air rise, like helium in balloons and billowing hot smoke.
We use this property to collect gases less dense than air by delivering them into an inverted gas jar. This is the upward delivery method.
3. Downward delivery: sink and never leave
Conversely, we collect gases denser than air using downward delivery .
This is the laziest method. We simply deliver the gas into a gas jar and let it sink.

4. Cheat sheet: list of common gases
We cannot collect soluble gases by the displacement of water. Instead, we must use upward delivery for soluble gases less dense than air, or downward delivery for soluble gases denser than air.
Study more leh:

Virtual lab: Identifying an unknown gas

Purifying water

The chemistry of aroma: Pandan smell in tap water
Leave a Reply Cancel reply
Privacy preference center, privacy preferences, discover more from chem not cheem.
Subscribe now to keep reading and get access to the full archive.
Type your email…
Continue reading
The Right Way to Make Hydrogen and Oxygen From Water

Introduction: The Right Way to Make Hydrogen and Oxygen From Water

In this Instructable, I will demonstrate one of the easiest and safest ways to make hydrogen and oxygen gas at home from water and baking soda, a process called Electrolysis. Non-Toxic.
Do not use table salt.
Step 1: Gather Materials...

For this experiment, you will need:
1. Water (Distilled water works best but tap water will work fine)
2. Cup/Jar/Container (Doesn't have to be anything specific, can use any container that will hold water, I used a beaker because that is what I had on hand)
3. Alligator Clips (To connect the electrodes to the battery, for this I made my own with wire and alligator clips, something I will show in the future)
4. Pencil Lead (Pencil Lead from mechanical pencils is easy to get and works good for small experiments, best to use carbon rods if you have them)
5. 9v Battery (For the power source)
6. Baking Soda (Water by itself is really bad at conducting electricity, so you add baking soda to act as an electrolyte and helps to conduct electricity through the water) ( many other tutorials say to use Table Salt - NaCl, this, however, is really bad because it produces extremely toxic Chlorine gas, so please NEVER use salt.
7. Tape (To hold the clips to the container)
Step 2: Add Baking Soda...

After you have filled your container with water, add about half a tablespoon of baking soda to the water and stir until water is clear.

Step 3: Attach Alligator Clips...

Rip/Cut a piece of tape about 2 inches long and cut it down the middle to make two strips.
Tape the alligator clips to the top of the container like in the picture.
MAKE sure the clips are not touching the water or touching each other.
Step 4: Insert Pencil Lead...

Once the alligator clips are secure, carefully insert the pencil lead into them.
Take care not to break the lead.
Step 5: Connect Clips to Battery...

Carefully connect the clips to the positive and negative terminals on the battery.
Take note of which clip is connected to the positive and negative.
If you have black and red clips/wire, black is negative and red is positive.
Step 6: Start Making H2 and O2!...

As soon as you connect the clips to the battery, you will immediately get bubbling to start on the pencil lead.
Oxygen will bubble from the electrode that is connected to the positive battery terminal.
Hydrogen will bubble from the negative.
Congrats, you have split the water molecule - H2O into H2 and O2 gas! with a bit of carbon dioxide from the baking soda.
This setup is more for viewing purposes and would be extremely hard to collect the gas like this. When you are done with the water you can pour it down the drain.
Step 7: If You Have Carbon Rods...

If you have carbon/graphite rods, this will work the same and you will get much bigger bubbles.
Have fun and remember, safety first!

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

10: Experimental Determination of the Gas Constant (Experiment)
- Last updated
- Save as PDF
- Page ID 93994

- Santa Monica College
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
- To experimentally determine the value of the Gas Constant, \(R\)
- To practice using the Gas Laws to solve a variety of problems.
A gas is the state of matter that is characterized by having neither a fixed shape nor a fixed volume. Gases exert pressure, are compressible, have low densities and diffuse rapidly when mixed with other gases. On a microscopic level, the molecules (or atoms) in a gas are separated by large distances and are in constant, random motion.
Four measurable properties can be used to describe a gas quantitatively: pressure (\(P\)), volume (\(V\)), temperature (\(T\)) and mole quantity (\(n\)). The relationships among these properties are summarized by the Gas Laws, as shown in the table below.
A closer look at the Combined Law reveals that the volume of a gas depends on both the pressure and temperature. Thus, if the volumes of two gases are to be compared, they must be under the same \(P\) and \(T\). A commonly used set of \(P\) and \(T\) reference conditions is known as Standard Temperature and Pressure, or STP. Standard temperature is defined as exactly 0 °C (273 K) and standard pressure is defined as exactly 1 atm (760 mmHg).
The Ideal Gas Law is obtained by combining Boyle’s Law , Charles’s Law and Avogadro’s Law together:
\[PV = nRT\]
Here, \(P\) represents as the gas pressure (in atmospheres); \(V\) is the gas volume (in Liters); \(n\) is the number of moles of gas in the sample; \(T\) is the gas temperature (in Kelvins). \(R\) is a proportionality constant called the Gas Constant, and has a theoretical value of 0.08206 \(\frac{L \cdot atm}{mol \cdot K}\). Note that the units of \(R\) will allow the units of \(P\), \(V\), \(n\) and \(T\) in the Ideal Gas Law to cancel correctly.
In this lab, students will measure various properties of a sample of hydrogen gas in order to experimentally determine the value of the Gas Constant, \(R\). The single displacement reaction between magnesium metal and hydrochloric acid will be used to generate the hydrogen gas:
\[\ce{Mg(s) + 2HCl(aq) -> MgCl2 (aq) + H2 (g)}\]
The hydrogen gas will be collected in a eudiometer , a tube closed at one end and marked in milliliter volume units. The gas will be collected in the closed end of the tube over a water bath via the technique of water displacement (see figures below)
Students will then obtain the following values for the collected sample of hydrogen gas: (1) Volume, (2) Temperature, (3) Moles, and (4) Pressure. The hydrogen volume will be directly measured from the eudiometer scale. The hydrogen temperature will also be directly measured using a thermometer. However, the mole quantity and pressure of the hydrogen gas must be determined indirectly. The mole quantity of the collected hydrogen can be easily calculated from the measured mass of the magnesium reactant using stoichiometry. But the hydrogen pressure is a little more difficult to obtain. Since hydrogen is collected over a water bath, a small amount of water vapor is mixed with the hydrogen in the eudiometer. The combined pressure of the \(\ce{H2}\) and \(\ce{H2O}\) gases will be equal (after adjustments) to the external atmospheric pressure:
\[P_{atm} = P_{hydrogen} + P_{water vapor}\]
\(P_{atm}\) (atmospheric pressure) will be measured using a barometer. \(P_{water vapor}\) (the partial pressure of water vapor) depends on the temperature of the water bath, and can be obtained from the table supplied below. By substituting these values in the above equation, the pressure of hydrogen \(P_{hydrogen}\)) will be determined.
Finally, to determine the value of the Gas Constant (\(R\)), the quantities \(V\), \(T\), \(n\) and \(P\) obtained for the hydrogen gas must simply be substituted into the Ideal Gas Equation. Students can then evaluate their accuracy in this experiment by comparing their experimental result to the true theoretical value of \(R\), and by calculating their percent error.
Materials and Equipment
4.0-cm ribbon of magnesium, length of copper wire (reusable), 6 M \(\ce{HCl}\) ( aq ), 50-mL eudiometer*, eudiometer stopper with hole(s)*, burette stand, large beaker, thermometer, small funnel, small graduated cylinder, barometer, large tub of water, electronic balance and sandpaper.
Concentrated \(\ce{HCl}\) is dangerous! Handle it with extreme care as demonstrated by your instructor. If any spills occur, inform your instructor immediately. Wash under running water (sink or shower) and use the neutralizing sodium bicarbonate solution supplied at the sinks if necessary. Also note that hydrogen gas is flammable, so be sure to have no open flames nearby when you perform this experiment.
Experimental Procedure
Magnesium Ribbon
- Obtain a 4.0-cm ribbon of magnesium (\(\ce{Mg}\)), a piece of sandpaper, and a length of copper wire.
- Carefully sand the outside of the \(\ce{Mg}\) ribbon to remove any oxide coating. Do not sand on the bench top! Place the \(\ce{Mg}\) ribbon on a paper towel while sanding. Weigh the cleaned \(\ce{Mg}\) ribbon and record this mass on your report form. Note that this mass should be less than 0.040 grams. If it is heavier, your \(\ce{Mg}\) ribbon will have to be “trimmed” by your instructor.
- Wrap the \(\ce{Mg}\) around the end of the copper wire. Do this in a tight ball with only a small gap between layers. Then wrap the copper wire to form a cage around the \(\ce{Mg}\) ball. The cage must be tight enough to keep the \(\ce{Mg}\) inside, but loose enough to allow water to easily flow around the wire. Roughly 3-cm of copper wire should be left over as a “handle” (see Figure 1).
Eudiometer Set Up and Reaction
- Obtain a eudiometer tube and stopper (with holes) from the stockroom. Use the burette clamp to hold it in place, open end up.
- Add ~10-mL of 6 M \(\ce{HCl}\) ( aq ) to the eudiometer tube using a small funnel. Then add tap water to the eudiometer carefully until it is filled to the brim (see Figure 1).
- Hang the \(\ce{Mg}\) ball inside the open end of the eudiometer, ~2-cm down from the top. Then insert the stopper into this end, and, while holding it in place, quickly invert the entire tube into your largest beaker 3⁄4 filled with water. Clamp the tube in the water in the upside down position (see Figure 2).
- The reaction will occur as soon as the acid diffuses down the tube and reaches the \(\ce{Mg}\) ribbon. As hydrogen gas is generated it will fill the eudiometer by forcing the water out of the tube and into the beaker via water displacement (see Figure 2). Allow the reaction to proceed until no \(\ce{Mg}\) is left and no further gas is formed. This should take 3-5 minutes.
Pressure Equalization
- To ensure that the pressure of hydrogen (and water vapor) in the eudiometer is equal to atmospheric pressure, the level of the water inside the tube must be the same as the level of water outside the tube. To achieve this, transfer both the tube and the beaker of water into the large bucket of water in the sink. Then raise or lower the tube until the internal and external water levels are equal.
Measurements
- After equalizing the water levels, record the following measurements:
- The volume of hydrogen gas collected (read directly from the eudiometer scale), in mL
- The temperature of the hydrogen gas collected, in °C. This can be measured by first removing the stopper then placing the thermometer directly in the eudiometer (keep the tube inverted so the gas does not readily escape). It is also acceptable to assume that the temperature of the hydrogen gas is the same as the temperature of the water bath, especially if you wait a while before making your measurements.
- The atmospheric pressure (use the lab barometer), in mmHg
- The temperature of the water in the plastic tub (use the thermometer), in °C
- The vapor pressure of water at the above temperature (obtain from Table on page 2), in mmHg
- When finished, repeat this entire procedure a second time with a fresh piece of magnesium ribbon.
Figure 1: Before Inverting
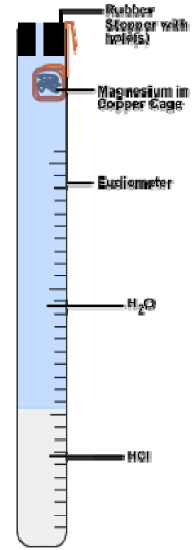
Figure 2: After Inverting

Pre-laboratory Assignment: Experimental Determination of the Gas Constant
- What is the name of the gas that will be collected and studied in this lab? Write the balanced equation for the reaction used to generate this gas.
- You will perform several measurements on your collected gas sample in order to experimentally determine the value of the Gas Constant (\(R\)).
- What is the theoretical value of \(R\), and what are its units?
- The Gas Constant is found in the Ideal Gas Law. Write the equation for this law.
- The magnesium ribbon used in this reaction must be carefully handled.
- What must you avoid doing with the magnesium ribbon at the lab bench (hint, see Procedure #2)?
- What mass of the magnesium ribbon should be used
- What is the name of the specialized "tube" that your gas is collected in?
- This tube not only collects the gas, it also allows you to directly measure the gas (circle one): pressure / temperature / volume
- Consider some of the other equipment you will use in this lab.
- What device will you use to measure atmospheric pressure?
- What TWO temperatures will you measure with your thermometer?
- Part of the procedure for this experiment involves ensuring that the total pressure of gases collected inside the specialized tube is equal to atmospheric pressure. How is this achieved (hint, see Procedure #8)?
Lab Report: Experimental Determination of the Gas Constant
Experimental Data
Data Analysis
Using your experimental data, determine the value of \(R\), the gas constant. Show all your conversions and calculations for each step clearly in the table below. Pay attention to units and significant figures.
- Average value of \(R\) (include units):
- Percent Error between your average value and the theoretical value of \(R\) (show work):
- The hydrogen generated in this lab was a product of the reaction between magnesium and hydrochloric acid. Which of these reactants was the limiting reactant? Provide experimental evidence to support your choice.
- Suppose when you inverted the eudiometer, a bubble of air became trapped inside it. Would this make your experimental value of \(R\) larger, smaller, or have no effect? Briefly explain your response.
- In Santa Monica, a sample of dry hydrogen gas inflates a balloon to 43.0 mL at 761 torr (sea-level). If the temperature remains unchanged, what is the balloon’s volume (in mL) in Denver, where the pressure is 12.2 psi (5000 ft elevation)? Assume that no gas has been added or removed.
- Another balloon is inflated to a volume of 1.250 L with dry hydrogen gas, at 28.0 °C. The balloon is then cooled, and its volume drops to 964 mL. If the pressure is unchanged, what is the hydrogen gas temperature (in °C) in the cooled balloon? Assume that no gas has been added or removed.
- Yet another balloon is inflated to a volume of 434 cm 3 using 0.141 moles of dry hydrogen gas. An additional 0.129 grams of hydrogen is then injected into the balloon at constant pressure and temperature. Calculate the new volume of the balloon (in cm 3 ).
- Hydrogen gas can be generated from the reaction between aluminum metal and hydrochloric acid:
\[\ce{2Al (s) + 6HCl (aq) -> 2AlCl3 (aq) + 3H2 (g)}\]
- Suppose that 3.00 grams of \(\ce{Al}\) are mixed with excess acid. If the hydrogen gas produced is directly collected into a 850. mL glass flask at 24.0 °C, what is the pressure inside the flask (in atm)?
- This hydrogen gas is then completely transferred from the flask to a balloon. To what volume (in L) will the balloon inflate under STP conditions?
- Suppose the balloon is released and rises up to an altitude where the temperature is 11.2 °C and the pressure is 438 mmHg. What is the new volume of the balloon (in L)?
Collection of Gas Over Water
In many cases, the amount of gas evolved by a reaction is of interest. Since gases have such small densities, it is usually not practical to collect the gas and find its mass. For gases that are not particularly soluble in water, it is possible to collect the evolved gas by displacement of water from a container.
The setup for the collection of a gas over water involves a container in which the reaction takes place and a gas collection container filled with water and inverted in a reservoir of water. The gas evolved from the reaction is collected by attaching one end of a hose to the reaction container and inserting the other up into the inverted gas collection bottle. As the gas is created, it will displace water from the bottle. The volume of gas can be determined by the amount of water that was displaced by the gas.
The volume of gas collected and the gas laws can be used to calculate the number of moles of gas collected.
During the collection, the water level in the container will adjust so that the pressure inside and outside the container are the same. Because of this, if we know the atmoshperic pressure, we also know the pressure of the gas inside the bottle.
The pressure inside the bottle is partially from the gas being collected and partially from the water vapor that has escaped from the surface of the water in the jar. The water inside the jar will reach an equlibruim state where the number of molecules leaving the surface is the same as the number returning. The equilibrium pressure of water is temperature dependent and is called the vapor pressure of water.
Dalton's Law of Partial Pressures tells us that the total pressure in the container must be the sum of the pressures of the gas we collected and the water vapor.
P T = P gas + P H 2 O
This equation can be used to calculate the pressure of the gas collected. Once the pressure of the collected gas is known, the number of moles of gas can be calculated using the ideal gas law:
- P = Pressure of the gas
- V = Volume of water displaced
- n = number of moles of gas
- R = the ideal gas constant
- T = the temperature of the gas
Navigating By Joy
Learning, laughing and loving together, chemistry for kids – how to separate water into hydrogen and oxygen using electrolysis.
We’ve all been told that water is made up of hydrogen and oxygen. But how do we really know that? Can this wet substance that quenches our thirst and cools our bodies on hot summer days really be made up of two gases ?
We tried to separate water into oxygen and hydrogen using electrolysis. We managed it after a series of experiments that left us with even more questions than we had before we started. Which isn’t necessarily a bad thing – curiosity is a great learning state! (See the mysterious case of the missing oxygen, below.)
You can benefit from our mistakes and perform electrolysis the quick way. Here’s how to split water into hydrogen and oxygen using electrolysis. Afterwards I’ll tell you about what we did first, which produced a different gas entirely.
How to separate water into hydrogen and oxygen
What you need.
- glass or plastic tub
- 2 elastic bands
- 2 test tubes (with lids if possible)
- bicarb of soda (1 tbsp)
- graphite pencil leads
- battery (we used 6V, a bit like this one )
- 2 pairs of crocodile clips
- waterproof tape
What you do
See this video for detailed set-up instructions – the elastic band arrangement keeps the test tubes in place perfectly.
If you can’t watch the video, here’s the gist of it: Connect one end of each crocodile clip to a piece of graphite, and the other to the battery. Secure the graphite ends to the bottom of the tub with the graphite sticking up, and place an inverted test tube over each piece of graphite (held in place by the elastic bands). Dissolve the bicarb of soda in the water and fill the tub. Finally, remove each test tube, fill it with the water, and carefully replace it over the graphite. Any gases collected during the electrolysis will replace the water in the tubes, so make sure there are no air bubbles.
What happens
Bubbles of gas quickly start to form at each electrode. More gas collects at the negative electrode (cathode) than at the positive (anode).
How to test your gases
When you’ve collected plenty of gas at each electrode, carefully put the lids on your test tubes (while they’re still underwater).
To test for hydrogen
We hypothesised that the gas at our (negative) cathode was (positively charged) hydrogen. Hydrogen is explosive. It won’t wreck your house in these quantities, but it will make a cool popping noise in the presence of a lighted splinter of wood. You can hear it in the video below.
To test for oxygen
We test for oxygen with a glowing splint. If enough oxygen is present, the splint rekindles. The gas we collected at our anode gave a brief glow which confirmed it to be oxygen, but after the excitement of the popping hydrogen, we were a bit disappointed. We produced much more oxygen later using a different method – see below for a video of our relighting splint.
How does electrolysis work?
Water is a covalent molecule (H20) held together by shared electrons in covalent bonds.
During electrolysis, the molecules are reduced at the cathode to to hydrogen gas, and oxidised at the anode to oxygen gas.
Pure water doesn’t conduct electricity, so we need to add an electrolyte, like bicarbonate of soda. (You wouldn’t believe the number of websites that tell you to use salt. We tried it, and collected a completely different gas. More on that later.)
Twice as much hydrogen as oxygen is produced, reflecting the molecular composition of water.
Here’s a fairly easy-to-follow explanation of the electrolysis of water .
If you’re looking for a more detailed explanation, see Wikipedia .
{Thank you so much, Sarah, for pointing out my earlier misunderstanding and for making this post more accurate!}
The mysterious case of the missing oxygen
(Or, what happens when you use salt as an electrolyte.)
Before we successfully split water into hydrogen and oxygen using the method above, we tried adding salt to help our water conduct electricity. And not just a pinch of salt. I decided that if a little salt would help a bit, then a lot of salt would be even better. (It works for crystals, after all.)
We set up our electrolysis using the same apparatus as above but this time with a saturated salt solution. And there we sat, eagerly looking for our bubbles of hydrogen and oxygen.
What happened? Well, plenty at our cathode. Gas quickly began to fill the test tube. We tested it and discovered it was hydrogen. And at the positive electrode? Not one single bubble of gas! What had happened to the oxygen from our water molecules?
I did a bit of research overnight.
It seems that during the electrolysis of sodium chloride (salt) solution , sodium chloride breaks down at the positive electrode to form chlorine gas and sodium hydroxide solution. (Click the link for a more detailed explanation.) Chlorine dissolves easily in water, so won’t collect as a gas until the solution is saturated and can absorb no more chlorine.
So if our positive electrode was busy attracting chlorine, and hydrogen was collecting at the cathode … what had happened to the oxygen? Or to the sodium from our sodium chloride (NaCl), for that matter? According to the chemists, the sodium and oxygen combine to make sodium hydroxide solution. Further investigation was called for.
We’d left our apparatus set up – disconnected from the battery – overnight. We decided to examine it for clues.
Further investigations
What changes had taken place as a result of electrolysis? Our salt solution had turned a brownish colour. Was this dissolved chlorine? Broken down graphite? Corroded crocodile clip (which had been attached to the anode)?
Filtering the solution . Some of our positive electrode (anode) broke down, leaving black bits in the solution. We use graphite in electrolysis because it is an inert (non-reactive) metal, but perhaps the large amounts of chlorine we produced had caused it to react? We filtered the brown solution to see if any insoluble bits remained. They didn’t. But we did notice some white spots on the filter paper – the chlorine produced at our positive electrode must have bleached the paper!
Testing the pH of the solution We hypothesised that the solution would be slightly alkali due to the sodium hydroxide. But when we tested it, we found the opposite. It was slightly acidic – like chlorine. We guessed this meant the solution must contain more chlorine than hydroxide.
More fun with oxygen
I’m going slightly off topic here, but I promised to say how we created enough oxygen to successfully test for it. We got the idea from going to The Magic of Oxygen show at the Royal Institution. I’d love to share with you one of the demonstrations we saw there.
The presenters asked me if they could borrow a £10 note from me – and then they set fire to it! Here’s a video of my flaming money.
The Magic of Oxygen scientists also demonstrated how to make “elephant toothpaste” by breaking down hydrogen peroxide. We remembered how we once made our own elephant toothpaste . When we got home we decided to make elephant toothpaste again, and use a glowing splint to test for oxygen gas.
When you place a glowing splint into oxygen, the splint re-lights.
Why this is my favourite way to do homeschool science
As you can tell, this was not the the kind of homeschool science demonstration where mum knows exactly what’s going to happen and why. I studied chemistry until I was sixteen – nearly thirty years ago! I didn’t know the answers to many of the questions generated by these experiments.
But not knowing what would happen made me curious and inspired to learn more, and the children were definitely caught up in my excitement. And I’m glad we made the “mistake” of using salt as an electrolyte first, because if we hadn’t we would have missed out on some very cool science!
Have you done any fun science recently?
Have you ever investigated a case of missing oxygen?
I’m appreciatively linking up here:
Weekly Wrap-Up – Weird Unsocialized Homeschoolers Collage Friday – Homegrown Learners The Home Ed Link Up #16 – Adventures in Home Education Science Sunday – Adventures in Mommydom Finishing Strong – Starts at Eight
The Hip Homeschool Hop – Hip Homeschool Moms
82 thoughts on “ Chemistry for kids – How to separate water into hydrogen and oxygen using electrolysis ”
Wow, this is really excellent, so much extension from a seemingly straightforward experiment! We did the very first part of the electrolysis, with slight variation from yours, and didn’t think to test the gas! I really like how you approach science!
Thank you so much, Hwee – you are very kind! I do love it when science unfolds like this did. It feels so easy when one is naturally caught up in the excitement!
You know how I love this kind of science fun!!
Thank you, Phyllis! I love reading about this kind of science fun on your blog, too!
You really are making science fun. These are subjects I figured were too mature/boring for my younger student but this gives me ideas on how to introduce it. I’m visiting from Weekly Wrap Up hop.
Nita, I did wonder if this would go over my kids’ heads but it was something I decided I wanted to do anyway, so any learning on their part was a bonus! Perhaps because of that, they go caught up in the fun and learned quite a bit too!
I love, love, love, love this! It’s awesome.
Thanks Ticia!
Great post! So in depth and yet so easy to understand.
Thank you, Carol. It was one of our favourites so far. As soon as I get hold of a giant bag of M&Ms we’ll be doing your recent science activities – they’ll complement this well!
Excellent experiment! We’ve done a year of fun chemistry but this is definitely going on our must-try list! Thanks for the detailed explanation.
Thanks, Tonia. I’m coming over to chemistry section of your blog for more ideas for fun experiments!
Wow! Will you come teach science to my kids?? This is amazing. I “Pinned” it for later.
LOL – anytime, Leslie – I love this stuff! Plus I just looked up Saint Paul and it looks like my kind of place. 😉 Thank you for the pin, too!
Your enthusiasm for science experiments is evident. I bet you and your kids have so much fun together. Do you enjoy science as much as your children? It certainly seems that way which is probably why your science demonstrations are so successful and mine aren’t!
I do like the look of that elephant toothpaste. Science provides lots of opportunities for interesting photos!
I think I probably do enjoy science at least as much as my children! Once I get around to doing it, that is… It’s so much easier to gather maths books, or pencils and paper to write stories, than it is to set up equipment for science. But once we’ve started, momentum definitely carries us forward. I remember being very inspired by your posts about Charlotte’s interest in chemistry.
It’s funny you mention the elephant toothpaste photos. I enjoyed taking those so much that after we’d finished the experiment, I put the camera memory stick in my pocket so I could look at the photos on my Mac during the children’s swimming lesson. But… when I went to get the memory stick out, it was gone. The only place I could think I might have dropped it was when we’d walked the dog before swimming. So after swimming I went back to the woods … and found the memory stick buried in mud, right where I’d parked the car! The photos on it have been pinned hundreds of times, and they’re near the top of a google image search for “elephant toothpaste.” C suggested I should give all our photos a spell in mud!
I love the way you do science. I need you to teach my guys their science. I keep procrastinating this term because Native Americans are so much more interesting!
Claire – I think you fitted more science into your germ study than I get round to in a year! And C(10) would love to do history at your place!
Nicely done! We’d done something similar a couple years ago – I love your set up! 🙂
Thank you, Eva!
Now that’s some COOL stuff there! We’ve done Elephant Toothpaste before. Kids loved that!
Elephant’s toothpaste is so much fun, isn’t it, Jessy? I must get some more hydrogen peroxide in so we can do it again – it’s such a great science standby!
This is an awesome post! I am pinning for my daughter for this school year. I would love it if you would link up at the Geeky Educational Link Up! http://www.morethanacouponqueen.com/search/label/Geeky%20Educational%20Link%20Up
Thank you so much, Meagan! And what a fabulous sounding link up – I would love to join in, thank you for the invitation!
Thanks for linking up over at the Geeky Educational Link Up. This is a GREAT post!
Thank you so much, Dawnita! And thanks for hosting the link up.
This is just awesome!!! I did this in high school chemistry…but it have never tried it with the kids at home – you’re very brave 🙂 And, all the follow-up!?! Just fantastic.
Thank you so much – you are very sweet!
Brave… foolish …?! I suspect a lot of the science I do with my kids goes over their heads, but they definitely get caught up in my excitement – which can’t be a bad thing, I hope! 😀
This is a good post…you light up some new idea in kids mind ,i am thinking how we can make H2 as a fuel for vehicles and industries….
Thank you! Yes there are lots of applications, aren’t there? We’ve had some interesting discussions stemming from this experiment.
Ok,Welcome,fine i would like to join your group regarding this,i completed my M.sc food science and technology..and now working in an MNC as QA supervisor come analyst..
That sounds like a very interesting course and job!
Yup ,sure its interesting ..me searching jobs in European countries..i heard that there are lots of vacancies… what about your carrier and family..I think you and your group trying to give knowledge to kids..am I right?
Very thanks……. Tell me.
I want to drive a water engine. So my helped.
Thanks for visiting!
Now how do you combine this electrolysis lab with understanding of molecules and atoms?
Hi Danielle, This tied in for us with our “ oxygen pancakes ” activity, in which we looked at the composition of a water molecule and hydrogen and oxygen atoms. (Using food, which always goes down well round here!)
Hello Ms.? or Mr.?
My Name is Daniel and I was wondering how do create oxygen with electrolysis without obtaining chlorine or and black pieces. Pls inform. I put a 9 volt battery in water and i had the same results. Pls help thanks.
Hi Daniel, When we used baking soda (bicarbonate of soda) as our electrolyte we didn’t produce chlorine or black pieces. We only got them when we performed electrolysis on a saturated salt solution. If you follow the procedure outlined at the start of this post you should be able to do the same. I hope this helps. Let me know how you get on.
Hello all.. This is Jewel..I have food technology background.Now I am working in Qatar,from my knowledge what I would like to share you all is ..water is the elixir of life…like our food washing,car washing…water can remove the poisonous material inside our body. The waste removal in our body is in mainly three ways 1.by Sweating 2.by urine 3.by fecal so if we drink more water we can remove maximum waste from our body,while summer time the water mainly goes through the urine and while cold its mainly by urine. So by god gift we can do wonders with water,may be that’s why god given water more to earth. Like fuel burning mechanism,oxygen should need for each process..so by water/oxygen/and good thinking we can do wonders…
Hello Jewel, Thank you for sharing that. Water really is the elixir or life! You’ve inspired me to go grab myself a glass. 🙂
Welcome dear friend..
Sorry While summer its mainly through sweating and less through urine..but cold or Rainy season it’s opposite..
what voltage are you supposed to use?
Thanks, Jesse! We used a 6 volt battery, like this one .
I had used a 9 volt battery. Thank you. I am going to try the elephant toothpaste.
Did the 9 volt battery work, Jesse?
I hope you enjoy elephant toothpaste!
Yes it worked. Why does some greenish foam float on the water?
Anyway the experiment was wonderful.
Hi Jesse, I’m so pleased your electrolysis worked! What did you use as your electrodes, and as your electrolyte? The greenish foam may have been a product of a reaction involving one of them?
Hi, I think this experiment is great, but I just want to point out that your explanation of the electrolysis of water is incorrect. You cited Wikipedia, but you have misinterpreted the information presented there. Water is NOT an ionic substance and the hydrogens and oxygen don’t simply pull apart and collect at the different electrodes. Water is a covalent molecule held together by shared electrons in the covalent chemical bonds. During electrolysis, the molecules are reduced at the cathode to hydrogen gas and oxidized at the anode to oxygen gas. That’s two different reactions going on, not one single splitting. Compounds like table salt are ionic. It dissociate into positive sodium and negative chloride when it dissolves. I recommend that you revisit your Wiki reference and revise your explanation or at least delete it. Try this resource for an easier to understand explanation: http://www.nmsea.org/Curriculum/7_12/electrolysis/electrolysis.htm
Hi Sarah, Thank you so much for taking the time to leave your comment, I appreciate it. I’m going to have a good look at that link and will update the post asap!
You’re welcome, Maqsood.
what to do if we cant get the test tubes
You could try a thin jar? You’d see the gas collecting, though you may struggle to contain it when you try to test it.
Seriously I never wonder we could intoduce chemistry’s conept for kids, but this one is look fun.
Hello madam Please help me. I separete hydrogen from water and make kit To conserve the hydrogen
Thank you for sharing this science experiment. I am using this for my science fair project.
Excellent , I hope your project went well! Thank you for taking the time to comment!
thank you! you saved me from the science fair in my school! i totaly make this Project ! grettings from Honduras!!!(sorry for my bad english)
Fantastic! I hope your project went well, Susana! Greetings to you in Honduras from Brighton, England 🙂
Your English is very good, by the way!
So I think this is a really cool project actually 2 of my kids did a similar project. Kyle my 12 year old boy did a how video games(fortnight) affect aggression you can see it at fortniteburger.net And Chad did a project on if waffles are good for you example blue waffles look more appetizing then regular waffle go to images and look up blue waffles yum yum. #ProudMom #AwesomeKids #LOL
Thanks Debra. your kids’ projects sound really interesting, you so should be proud of your awesome kids !
Is there a video of the whole process when you create the model?
Hi Theo I’m afraid not, we did this several years ago and didn’t think to video the set up. I wish I had!
“We produced much more oxygen later using a different method – see below for a video of our relighting splint.”
What was this other method, please?
Hi Gary, I confess I can’t remember what I was referring to now – we did this a few years ago. But I know we made lots of oxygen from making elephant toothpaste so you could try that. Or one of my children made oxygen using liver and hydrogen peroxide – see the YouTube link below.
http://navigatingbyjoy.com/2013/02/16/elephants-toothpaste-fun-with-catalysts/
https://youtu.be/_Lk9BD0z9zI
this suck! i did not work!!! i did everything! it still did not work. Do not do this!!!
Sorry to hear the experiment didn’t work for you, Jewle. We’ve had some experiences like that too. It’s frustrating when you follow all the instructions but don’t get the result, isn’t it?
Hey Lucinda, thank you, the project was amazing. So i tried this out for myself and i did everything but for oxygen test tube it only got like very little oxygen and then it just stopped and wasn’t working after a few hours i check on it again but it was exactly the same so when decided to remove the test tube all the water got out of it and this yellowish stuff came out. My crocodile clip was also corroded and I was surprised. The hydrogen test tube was fine as nothing happened to it. Can you please help or explain to me why this happened and put me back on the right track? Thank you.
thank you for the article, the information helped me, success always
Hi,i liked your experiment it was vry funto make
Glad to hear that Wendy. Thank you for taking the time to leave a comment!
Hello! Im an 8th grader doing this project for honor science and right now I’ve gotten the whole process going but instead of bubbles in the negative cord it immersed turned brownish and the positive is doing alright and creating bubbles, am I doing something wrong? Please let me know before nov 4 thank you
Sorry I meant immediately turned brown*
I’m near 68 years old, and retited by disabilties, but I do love secince! and doing such experiments are fun still to me! I was a rhvac tech, I did repairs of heating & air condition equipment [residentual]. I remember this making fire from water as an experiment that the science teacher [in junor high did] it was a real attention getter. He told us, [it was called the brown expermint as I recall but that was not the man who first did the experiment it was called that becuse the durring the act of electrolysiss, the water turned brown from the inter action between the voltage applied and the electrolate]. doing this experiment in from of the class…………made him a rock star!! I have always wondered, what are the flue gases coming from burning the h20?? I have asked people who I though might know!, but I nrver did find out. I firgure, that using salt [as the elecyroyate] would put off a chemical by product by product, along with the h20 [hydrogen/oxygen] and anything appling 12 volts dc would do to the end product of conbustion. In closimg I just want to say I hope the young one seeing this experiment done in school get as moved as I did! this was the thing that made me want to know more about stuff. wanting to know more is what made me want to know more about about the device of the world. how does you refrigeratoor keep the milk cold how does the furnace heat the house. how does that gas engine on the lawnmower/lawn tractor work and when you turn a light in a house what actual happens! I must thank all teacher for planting the seads in our younger generation!!! It looks like it 3:30 am, part of the many things is I don’t sleep very good. but yyou just got to do the best with what you have. I keep pushing on because, somebody, somewhere has it worst and God bless them 606
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Notify me of follow-up comments by email.
Notify me of new posts by email.

IMAGES
COMMENTS
Sep 21, 2022 · Gas Collection by Water Displacement. Gases that are produced in laboratory experiments are often collected by a technique called water displacement (see figure below). A bottle is filled with water and placed upside-down in a pan of water. The reaction flask is fitted with rubber tubing which is then fed under the bottle of water.
Dec 7, 2024 · Water can be poured and takes the shape of the container it is in. Gas: In a gas, the particles move freely from one another. You can also say they vibrate! Gas particles spread out to take the shape of the container they are put in. Steam or water vapor is an example of a gas. Try This Free States of Matter Activity!
Dec 5, 2024 · From Water to Fuel: Simple HHO Gas Experiment You Must See!Discover the fascinating science of turning water into gas in this simple yet exciting HHO gas exp...
Nov 22, 2021 · We use this property to collect gases less dense than air by delivering them into an inverted gas jar. This is the upward delivery method. 3. Downward delivery: sink and never leave. Conversely, we collect gases denser than air using downward delivery. This is the laziest method. We simply deliver the gas into a gas jar and let it sink.
6. Baking Soda (Water by itself is really bad at conducting electricity, so you add baking soda to act as an electrolyte and helps to conduct electricity through the water) ( many other tutorials say to use Table Salt - NaCl, this, however, is really bad because it produces extremely toxic Chlorine gas, so please NEVER use salt. 7.
Sep 22, 2021 · The gas will be collected in the closed end of the tube over a water bath via the technique of water displacement (see figures below) Students will then obtain the following values for the collected sample of hydrogen gas: (1) Volume, (2) Temperature, (3) Moles, and (4) Pressure.
The equilibrium pressure of water is temperature dependent and is called the vapor pressure of water. Dalton's Law of Partial Pressures tells us that the total pressure in the container must be the sum of the pressures of the gas we collected and the water vapor. P T = P gas + P H 2 O. This equation can be used to calculate the pressure of the ...
First, test the gas in the test tube that has more gas in it. It should be the one over the negative electrode. If both tubes are completely filled with gas, make sure you look at the battery to see which tube is over the negative electrode. Put on your goggles. Light the wooden splint on fire (see left photo below).
Dec 5, 2024 · As the gas pushed out the water, it is pushing against the atmosphere, so the pressure inside is equal to the pressure outside. Gas Collection by Water Displacement. Gases that are produced in laboratory experiments are often collected by a technique called water displacement (see Figure below). A bottle is filled with water and placed upside ...
Sep 18, 2014 · Dissolve the bicarb of soda in the water and fill the tub. Finally, remove each test tube, fill it with the water, and carefully replace it over the graphite. Any gases collected during the electrolysis will replace the water in the tubes, so make sure there are no air bubbles. What happens. Bubbles of gas quickly start to form at each electrode.