- Methodology article
- Open access
- Published: 24 October 2018

Efficient transposon mutagenesis mediated by an IPTG-controlled conditional suicide plasmid
- Santa S. Naorem 1 na1 ,
- Jin Han 1 na1 ,
- Stephanie Y. Zhang 1 ,
- Junyi Zhang 1 ,
- Lindsey B. Graham 1 ,
- Angelou Song 1 ,
- Cameron V. Smith 1 ,
- Fariha Rashid 1 &
- Huatao Guo ORCID: orcid.org/0000-0002-2396-1934 1
BMC Microbiology volume 18 , Article number: 158 ( 2018 ) Cite this article
9186 Accesses
7 Citations
1 Altmetric
Metrics details
Transposon mutagenesis is highly valuable for bacterial genetic and genomic studies. The transposons are usually delivered into host cells through conjugation or electroporation of a suicide plasmid. However, many bacterial species cannot be efficiently conjugated or transformed for transposon saturation mutagenesis. For this reason, temperature-sensitive ( ts ) plasmids have also been developed for transposon mutagenesis, but prolonged incubation at high temperatures to induce ts plasmid loss can be harmful to the hosts and lead to enrichment of mutants with adaptive genetic changes. In addition, the ts phenotype of a plasmid is often strain- or species-specific, as it may become non- ts or suicidal in different bacterial species.
We have engineered several conditional suicide plasmids that have a broad host range and whose loss is IPTG-controlled. One construct, which has the highest stability in the absence of IPTG induction, was then used as a curable vector to deliver hyperactive miniTn5 transposons for insertional mutagenesis. Our analyses show that these new tools can be used for efficient and regulatable transposon mutagenesis in Escherichia coli , Acinetobacter baylyi and Pseudomonas aeruginosa . In P. aeruginosa PAO1, we have used this method to generate a Tn5 insertion library with an estimated diversity of ~ 10 8 , which is ~ 2 logs larger than the best transposon insertional library of PAO1 and related Pseudomonas strains previously reported.
We have developed a number of IPTG-controlled conditional suicide plasmids. By exploiting one of them for transposon delivery, a highly efficient and broadly useful mutagenesis system has been developed. As the assay condition is mild, we believe that our methodology will have broad applications in microbiology research.
Transposon mutagenesis is a powerful technique for bacterial genetic and genomic studies. One of the most widely used transposons is derived from Tn5. The Tn5 transposon contains two IS50 elements as inverted terminal repeats (Additional file 1 : Figure S1) [ 1 , 2 ]. Both IS50 and Tn5 can be mobilized by their encoded transposase (Tnp) protein, which recognizes two 19 base pair (bp) sequences at their ends, namely outside end (OE) and inside end (IE), for transposition [ 2 ]. OE and IE differ by 7 bp (Additional file 1 : Figure S1). As Tn5 insertion is almost completely random, it can insert into any gene in a bacterium. The native Tn5/IS50 is not very active, thus avoiding overt deleterious effect on their hosts, but hyperactive mutants have been engineered as genetic manipulation tools [ 2 , 3 ]. The most active one contains a mosaic sequence of OE and IE (mosaic end; ME) at the transposon termini and an engineered tnp gene encoding a highly active transposase enzyme (Tnp H ), which together increase Tn5 transposition by more than 1000-fold.
Transposons for insertional mutagenesis are usually delivered into bacteria through conjugation of a suicide plasmid [ 4 , 5 , 6 ]. Insertion mutants are then selected as the transposons are tagged with an antibiotic-resistance gene. The success of a transposon mutagenesis assay, especially a saturation mutagenesis assay, requires generation of an insertion library with high diversity, which requires efficient plasmid conjugation and transposon transposition. However, conjugation is inefficient in many bacterial species. Occasionally, electroporation has also been used to deliver transposon-containing suicide plasmids for mutagenesis, but low library diversities were often achieved using such approaches [ 7 , 8 , 9 ]. To perform efficient transposon mutagenesis in these organisms, temperature-sensitive ( ts ) plasmids are sometimes used for transposon delivery [ 10 , 11 , 12 , 13 , 14 , 15 , 16 ]. However, many organisms do not have a ts and easily manipulatable plasmid, and sometimes a ts plasmid in one organism is either non- ts or suicidal in a different organism [ 10 , 14 , 17 ]. In addition, a high temperature is often required to cure the ts plasmids after mutagenesis, which can be inhibitory to cell growth and may result in selection of mutants with adaptive genetic changes [ 10 , 11 , 14 ].
In this study, we have developed an efficient and regulatable transposon mutagenesis tool that exploits an IPTG-controlled conditional suicide plasmid. It contains an RSF1010 replicon, an IncQ-type replication origin that allows plasmid replication in most Gram-negative bacteria, as well as a few Gram-positive bacteria [ 18 ]. It is relatively small, so it can be easily modified. To control plasmid replication by IPTG, a second copy of the plasmid-encoded repF repressor gene is cloned downstream of the Escherichia coli tac promoter. For efficient and regulatable transposon mutagenesis, we used miniTn5 (mTn5) transposons and cloned the hyperactive transposase gene downstream of a lac promoter. We show that the resulting constructs can be used for efficient insertional mutagenesis in three different bacterial species. In Pseudomonas aeruginosa PAO1, we show that our system is able to generate a Tn5 insertion library that is almost 2 logs larger than the best library of PAO1 and related Pseudomonas strains previously reported, demonstrating that we have developed a powerful mutagenesis tool that is highly useful for microbiology studies.
Construction of IPTG-controlled suicide plasmids
To develop a method for efficient transposon mutagenesis in bacterial species that are difficult to transform and conjugate, we created multiple IPTG-controlled suicide plasmids that have a broad host range (Fig. 1a ). The plasmids were derived from pMMB208, which is a conjugatable plasmid containing an RSF1010 oriV (an IncQ-type origin of replication) that can replicate in most Gram-negative bacteria and a few Gram-positive bacteria [ 18 ]. Plasmid replication requires three proteins, RepA, MobA/RepB and RepC, which are a helicase, a primase and an oriV -binding protein, respectively. repF encodes a small repressor protein that binds the P4 promoter and controls the repF - repA - repC operon through feedback inhibition [ 19 , 20 ]. pMMB208 also contains a tac promoter (P tac ), a lacI Q gene and a chloramphenicol resistance marker ( Cam R ). To create a conditional suicide plasmid (pMMB- repF ), a second copy of the repF gene was inserted downstream of P tac . Upon IPTG induction, efficient plasmid loss from transformed E. coli DH10B cells was observed (99.97%; Fig. 1b ). As an alternative strategy, we inserted two repA helicase dominant negative mutants, K42A and D139A, downstream of P tac [ 21 ]. Similarly, IPTG was able to induce efficient plasmid loss from the transformed DH10B cells. In fact, plasmid retention rates of the dominant negative mutants (K42A, 4.9 × 10 − 7 ; D139A, 1.5 × 10 − 5 ) were much lower than that of pMMB- repF (3.5 × 10 − 4 ) (Fig. 1b ). However, the two repA dominant negative mutants showed significantly lower plasmid stability in the absence of IPTG induction (Fig. 1b ), suggesting that plasmid replication is strongly inhibited by leaky expression of the dominant negative mutants, or by spontaneous recombination of the wild-type and the dominant negative repA genes (~ 861 bp direct repeats). Consistent with that, there were ~ 20–30-fold less plasmid isolated from the same amount of cells for the two mutant constructs (Fig. 1c ). Therefore, we decided to choose pMMB- repF for further experiments. To kill the cells that still retain the plasmid after IPTG induction, we inserted a sacB counter selection marker into the vector [ 22 ], resulting in pMMB- repF / sacB . Indeed, insertion of sacB allows efficient killing of plasmid-containing cells by sucrose (data not shown; also see below).
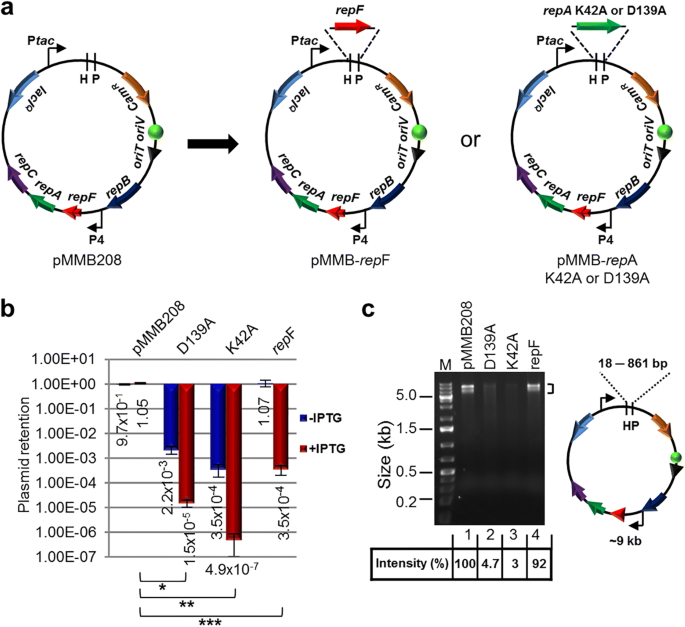
IPTG-controlled conditional suicide plasmids. a Plasmid pMMB208 and its conditional-suicide derivatives. pMMB208 contains an RSF1010 oriV for replication and an oriT for conjugation. Genes repA , mobA / repB and repC encode proteins required for plasmid replication, and repF encodes a transcription repressor that binds promoter P4. pMMB208 also has Cam R and lacI Q genes and a P tac promoter. Plasmid pMMB- repF is a derivative of pMMB208 that has a second copy of the repF gene inserted downstream of P tac . Plasmids pMMB- repA K42A and pMMB- repA D139A have a dominant-negative repA mutant gene, either K42A or D139A, inserted downstream of P tac . b Amount of E. coli DH10B cells retaining the indicated plasmids after 24 h growth in the absence of antibiotics, either with or without IPTG induction. Results were average of three independent experiments, and bars represent mean ± SD (standard deviation). * p < 0.0001, ** p < 0.0001, and *** p < 0.0001 by unpaired Student’s t-test for IPTG induced cultures. c pMMB208 and its derivatives are digested with Hin dIII (H) and Pst I (P). Comparing to pMMB208 and pMMB- repF , the repA K42A and D139A mutants showed reduced yields in plasmid minipreps (no IPTG induction; 3.0% and 4.7% of that of pMMB208, respectively). Hin dIII and Pst I digestion generates two fragments for each plasmid. The ~ 9 kb fragment is seen on the gel, while the shorter ones, ranging from 18 bp for pMMB208 to 861 bp for the repA mutants, are not visible. Another large band (~ 9 kb) is also seen in restriction digestion of pMMB208 and its derivatives, even after complete digestion, and the cause is unknown
IPTG-controlled mutagenesis of E. coli by a highly-active mTn5 transposon
A Kan R -tagged mTn5 was then inserted in pMMB- repF / sacB for transposon mutagenesis (Fig. 2a ) [ 4 ]. The mTn5 contains an OE and an IE at the termini. In addition, it contains an uncoupled, lac promoter (P lac )-controlled tnp H gene encoding the hyperactive transposase (Tnp H ) [ 3 ], thus allowing inducible expression of Tnp H . E. coli cells transformed with this plasmid, pSNC-mTn5, were cultured in LB media with and without IPTG induction for 24 h. Cells were then analyzed for efficiencies of plasmid loss, sucrose counter selection and transposon insertion (See Methods ). The plasmid is stable without IPTG induction, as ~ 91.4% of cells retained the plasmid (Cam R ) after 24 h culture in the absence of antibiotics (Fig. 2b ). In contrast, ~ 2.6 × 10 − 3 of the cells retained the plasmid post IPTG induction, suggesting that overexpression of the RepF repressor caused efficient plasmid loss. Sucrose counter selection further reduced plasmid-bearing cells (~ 1.6 × 10 − 6 are Cam R ; ~ 1600-fold reduction). In comparison, the percentage of Suc R Kan R cells after IPTG induction was found to be ~ 2.3 × 10 − 4 (Tn5-containing), significantly higher than that of Suc R Cam R cells (~ 1.6 × 10 − 6 , plasmid-containing), suggesting that Tn5 transposition had occurred efficiently (Suc R Kan R Cam S : ~ 2.3 × 10 − 4 ). Colony restreaking showed that 150/150 Suc R Kan R colonies were Kan R Cam S (Fig. 2c ). Colony PCRs, which used two sets of primers (P1 + P2 for detection of Kan R , or mTn5, and P3 + P4 for detection of tac - repF , or plasmid), confirmed plasmid loss in 10 out of 10 colonies (10/10) (Fig. 2d ). Sequence analysis showed that all 13 Suc R Kan R colonies analyzed had different Tn5 insertion sites (Fig. 3a ).
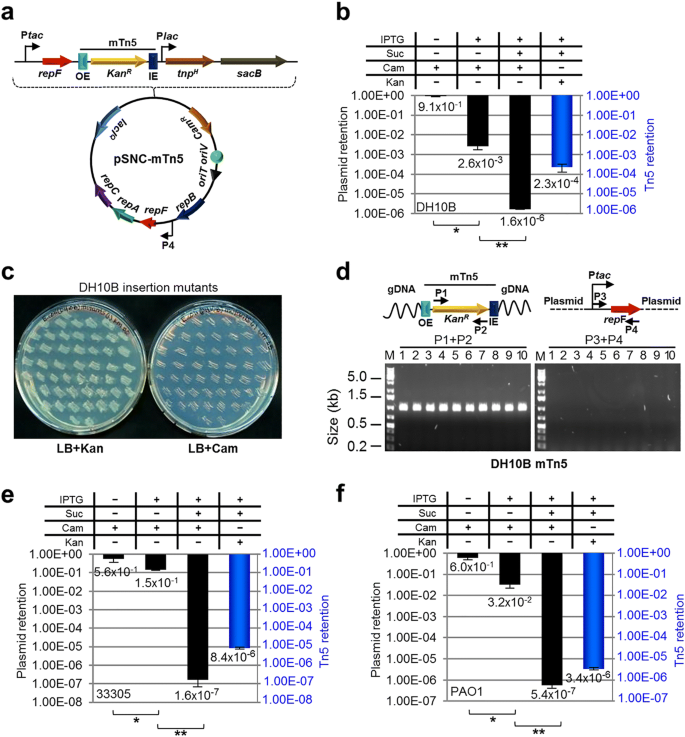
mTn5 transposon mutagenesis using an IPTG-controlled conditional suicide plasmid. a Diagram of plasmid pSNC-mTn5. pSNC-mTn5 is a derivative of pMMB-repF that contains a Kan R -tagged mTn5, a lac promoter-controlled hyperactive transposase gene ( tnp H ), and a sacB counter selection marker (with its own promoter). OE and IE are outside and inside ends of the mTn5. b Plasmid and transposon retention frequencies in E. coli DH10B. A “+” symbol for IPTG indicates that the inducer was added to the liquid culture, and a “+” symbol for Suc, Cam, and Kan indicates that the chemicals were added to the plates. Black columns represent plasmid retention frequencies, and the blue column represents Tn5 retention frequency. Results were average of three independent experiments, and bars represent mean ± SD (*p < 0.0001 and ** p = 0.0054 by unpaired t-test). (see Methods for details) ( c ) Colony restreaking. 150/150 Suc R Kan R colonies of DH10B were found to be Kan R Cam S and 50 are shown here. d Colony PCR of 10 restreaked clones in ( c ). Primer sets P1&P2 and P3&P4 detect Kan R and repF , respectively. All were mTn5-positive and plasmid-negative. Primers P3 and P4 are a functional pair for PCR-amplification of the plasmid sequence (data not shown). e Plasmid and transposon retention frequencies in A. baylyi. Results were average of three independent experiments, and bars represent mean ± SD (* p = 0.024 and **p < 0.0001 by unpaired t-test). f Plasmid and transposon retention frequencies in P. aeruginosa . Results were average of three independent experiments, and bars represent mean ± SD (* p = 0.0013 and ** p = 0.0038 by unpaired t-test). Colony restreaking and PCR analysis are shown in Additional file 2 : Figure S2
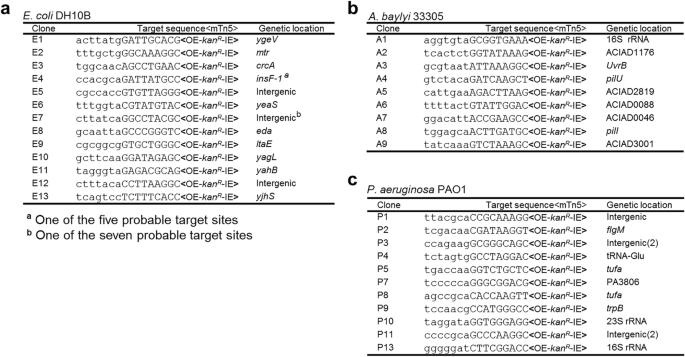
mTn5 insertion sites in different bacteria. a mTn5 insertion sites in E. coli DH10B. b mTn5 insertion sites in A. baylyi 33,305. c mTn5 insertion sites in P. aeruginosa PAO1. Only the chromosomal sequences next to the OE are shown. The 9 bp duplicated sequences are shown in capital letters. Identical clones are shown only once, with numbers indicated in parenthesis. Either gene names or locus tags are given as genetic locations
Efficient mutagenesis of Acinetobacter baylyi and P. aeruginosa by a highly-active mTn5 transposon
Construct pSNC-mTn5 was then tested in two Gram-negative, capsule-bearing bacteria, A. baylyi 33,305 and P. aeruginosa PAO1 [ 23 , 24 ]. Comparing to E. coli DH10B, transformed A. baylyi 33,305 and P. aeruginosa PAO1 appeared to lose the plasmid more easily in the absence of IPTG, with ~ 56.3% of A. baylyi and ~ 59.6% of P. aeruginosa retaining the plasmid after 24 h culture in LB media without antibiotics (Fig. 2e, f ). Following IPTG induction, ~ 14.7% of A. baylyi and ~ 3.2% of P. aeruginosa retained the plasmid, suggesting that IPTG induced additional plasmid loss from these organisms, although their efficiencies were lower than that in DH10B cells. With sucrose counter selection, ~ 1.6 × 10 − 7 of A. baylyi remained Cam R , indicating that they contained the plasmid (Fig. 2e ). Similarly, ~ 5.4 × 10 − 7 of P. aeruginosa cells were found to be Suc R Cam R (Fig. 2f ). These results suggest that IPTG and sucrose both contributed in reducing plasmid-bearing cells. In comparison, the percentages of Suc R Kan R cells were 8.4 × 10 − 6 for A. baylyi and 3.4 × 10 − 6 for P. aeruginosa , suggesting that Tn5 transposition occurred in both organisms prior to plasmid loss. Colony restreaking showed that 100/100 Suc R Kan R colonies are Suc R Cam S , suggesting that efficient plasmid loss had occurred following Tn5 transposition (~ 100% for both; Additional file 2 : Figure S2a, c). Loss of plasmids was further confirmed by PCR tests (Additional file 2 : Figure S2b, d). As observed in DH10B cells, Tn5 insertion also seemed to be random, as 9/9 A. baylyi and 13/15 P. aeruginosa mutants had different Tn5 insertion sites (Fig. 3b, c ). The detection of identical mutants suggests that cell growth ensued following transposon transposition (Fig. 3c ), which is common in different transposon mutagenesis assays [ 5 , 6 , 25 ].
Construction of a Tn5 insertion library of P. aeruginosa using the highly-active mTn5 transposon
To determine whether we can construct a transposon insertion library of P. aeruginosa PAO1 with high diversity, ten pSNC-mTn5 transformants of the bacterium were cultured independently and then combined and induced with IPTG to initiate transposon mutagenesis. Following 24 h culture in LB media containing IPTG, ~ 6.4% of cells retained the plasmid (Additional file 3 : Figure S3a). The frequencies of Suc R Cam R and Suc R Kan R cells in the IPTG-induced culture were found to be ~ 6.5 × 10 − 7 and ~ 3.5 × 10 − 6 , respectively. Based on the total number of cells cultured and the frequency of Suc R Kan R Cam S cells, the total diversity of the mTn5 insertion library was estimated to be ~ 1.3 × 10 7 , which covers the entire gene repertoire (5697) of P. aeruginosa PAO1 by ~ 2238 times [ 24 ]. To our knowledge, the diversity of this transposon insertion library is bigger than the best transposon insertion library of PAO1 and related strains previously reported (Table 1 ) [ 5 , 6 , 9 , 26 , 27 , 28 , 29 , 30 , 31 , 32 ]. Colony restreaking and PCR tests confirmed plasmid loss in the mutants (Additional file 3 : Figure S3b, c), and 28/37 clones analyzed had different Tn5 insertion sites (Additional file 3 : Figure S3d). Based on the percentage of independent clones in the library, its diversity is re-estimated to be ~ 1.0 × 10 7 .
An mTn5 with MEs enables generation of a P. aeruginosa mutant library with even higher diversity
To determine whether the efficiency of mTn5 transposition can be further improved, we replaced both OE and IE of the mTn5 with MEs (Fig. 4a ). The new plasmid, pSNC-mTn5ME, was transformed into DH10B cells. Cell growth (or colony sizes) appeared to be normal, suggesting that basal-level transposition, if any, did not lead to obvious cellular toxicity, which was our initial concern. The behavior of the plasmid and Tn5 transposition efficiency were determined under the same conditions described above. Without IPTG induction, the plasmid remained relatively stable, as ~ 100% of the cells retained the plasmid (Cam R ). After IPTG induction for 24 h, 1.1 × 10 − 3 of the cells retained the plasmid, suggesting that RepF overexpression caused efficient plasmid loss. With sucrose counter selection, ~ 2.4 × 10 − 5 cells remained Suc R Cam R . Interestingly, the frequency of Suc R Kan R cells was found to be very high (~ 28.0%), indicating that mTn5ME is much more active than the non-ME version (~ 1200 folds). Restreaking of Suc R Kan R colonies showed that they were all Kan R Cam S (100/100) (Additional file 4 : Figure S4a), and colony PCRs confirmed plasmid loss (10/10) (Additional file 4 : Figure S4b). Sequence analysis of the transposon insertion junctions showed that all 13 Suc R Kan R colonies analyzed had different Tn5 integration sites (Additional file 4 : Figure S4c).
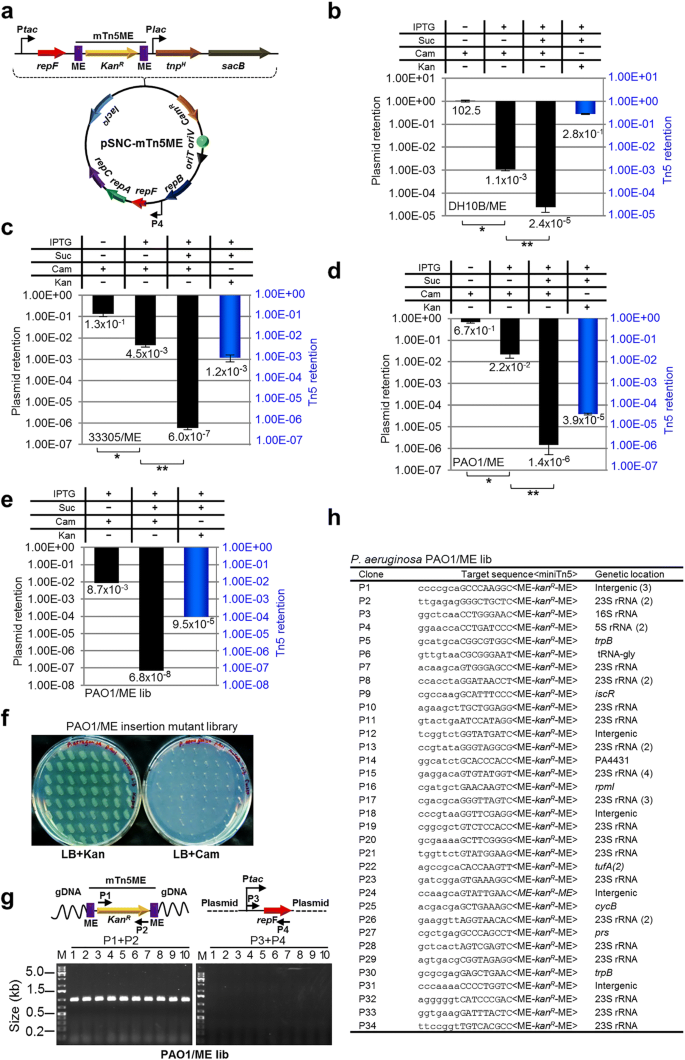
Generation of a P. aeruginosa insertion library with pSNC-mTn5ME. a Diagram of pSNC-mTn5ME, a derivative of pSNC-mTn5 that has MEs instead of OE and IE at the termini of mTn5. b Plasmid and transposon retention frequencies in E. coli DH10B. Results were average of three independent experiments, and bars represent mean ± SD (* p < 0.0001 and ** p = 0.0004 by unpaired t-test). Colony restreaking and PCR assays are shown in Additional file 4 : Figure S4. c Plasmid and transposon retention frequencies in A. baylyi . Results were average of three independent experiments, and bars represent mean ± SD (* p = 0.0029 and ** p = 0.0006 by unpaired t-test). Colony restreaking and PCR assays are shown in Additional file 5 : Figure S5. d Plasmid and transposon retention frequencies in P. aeruginosa PAO1. Results were average of three independent experiments, and bars represent mean ± SD (* p < 0.0001 and ** p = 0.0065 by unpaired t-test). Colony restreaking and PCR assays are shown in Additional file 6 : Figure S6. e Plasmid and transposon retention frequencies in the P. aeruginosa PAO1 mutant library generated with pSNC-mTn5ME. f Colony restreaking. 100/100 Suc R Kan R colonies of the mTn5ME library of P. aeruginosa were found to be Kan R Cam S . 50 are shown here. g Colony PCR of ten restreaked clones in ( f ) with the indicated primers. All were mTn5-positive and plasmid-negative. h Transposon insertion sites of 46 mutant clones from the mTn5ME insertion library of P. aeruginosa . Identical clones are shown only once, with their duplication numbers indicated in parenthesis
We then determined whether construct pSNC-mTn5ME would also be more active in A. baylyi 33,305 and in P. aeruginosa PAO1. For A. baylyi , the efficiencies of plasmid loss were higher for pSNC-mTn5ME than for pSNC-mTn5, both in the absence and presence of IPTG induction (Figs. 2e and 4c ). Sucrose counter selection was highly effective for both constructs (Figs. 2e and 4c ). As in E. coli , mTn5ME was found to be much more active than the non-ME version (~ 140 fold higher; Suc R Kan R cells: ~ 1.2 × 10 − 3 for mTn5ME vs. ~ 8.4 × 10 − 6 for mTn5). Similarly, colony restreaking of Suc R Kan R cells showed that 100/100 colonies are Kan R Cam S (Additional file 5 : Figure S5a), and plasmid loss was further verified by PCR (Additional file 5 : Figure S5b). 9/14 colonies were found to have different Tn5 insertion sites (Additional file 5 : Figure S5c). For PAO1, efficiencies of plasmid loss (±IPTG) were found to be similar for both pSNC-mTn5 and pSNC-mTn5ME (Figs. 2f and 4d ), and sucrose counter selection was also effective for pSNC-mTn5ME (Fig. 4d ). As in E. coli and in A. baylyi , mTn5ME was found to be more active than mTn5 in PAO1 (~ 11 fold higher; Suc R Kan R cells: ~ 3.9 × 10 − 5 for mTn5ME vs. ~ 3.4 × 10 − 6 for mTn5) (Figs. 2f and 4d ). In the colony restreaking assay, 100/100 Suc R Kan R colonies were found to be Kan R Cam S (Additional file 6 : Figure S6a), and PCR assays further confirmed plasmid loss (10/10) (Additional file 6 : Figure S6b). Sequence determination showed that 7/10 colonies tested had different Tn5 insertion sites (Additional file 6 : Figure S6c).
We then determined whether pSNC-mTn5ME would be a better construct than pSNC-mTn5 for transposon saturation mutagenesis in P. aeruginosa . Ten transformants were randomly picked for Tn5 insertion library construction using the protocol described above. About 0.87% of cells retained the plasmid after IPTG induction, and the frequency of Suc R Cam R cells was found to be 6.8 × 10 − 8 . In comparison, the frequency of Suc R Kan R cells was found to be ~ 9.5 × 10 − 5 , suggesting that efficient mTn5ME transposition has occurred. Based on the total amount of cells cultured and the mTn5ME transposition efficiency (~ 9.5 × 10 − 5 ), the diversity of the mTn5ME insertion library was estimated to be 1.02 × 10 8 , which is ~ 3 logs larger than the best PAO1 transposon insertion library previously reported and ~ 2 logs larger than a Tn5 insertion library of P. aeruginosa MPAO1, a derivative of PAO1 with ~ 0.2% genetic variation [ 6 , 33 ]. This new library is by far the biggest transposon insertion library of PAO1 and related species ever reported (Table 1 ). The size of our new library is enough to cover the entire gene repertoire of PAO1 by ~ 18,000 times. Colony restreaking (100) and PCR tests (10) confirmed plasmid loss in all the Suc R Kan R clones analyzed (Fig. 4f, g ), and 34/46 clones tested had different Tn5 insertion sites (Fig. 4h ). Thus, the independent clones in the library (library diversity) are estimated to be ~ 7.5 × 10 7 .
We have developed a new transposon mutagenesis system that is efficient, regulatable, easy-to-use, and broadly useful. We believe it will be especially useful for functional genomics studies of Gram-negative bacteria that are difficult to transform and conjugate, such as certain capsule-containing bacteria, obligate anaerobes, and possibly obligate intracellular pathogens as well. The advantage of this method relies on the following features: (i) A broadly-functional plasmid replicon; (ii) Replication of the plasmid is regulated by IPTG; (iii) The inclusion of the sacB gene for counter selection; (iv) A highly-active/hyperactive transposon; (v) Regulatable expression of the hyperactive transposase gene; (vi) mTn5 and mTn5ME transposons insert almost completely randomly in different bacteria (Additional file 7 : Figure S7) [ 34 , 35 ]. In addition, the relatively small sizes of the RSF1010-based plasmids also facilitate their transformation and conjugation. Similar to transposon mutagenesis using ts plasmids, our system does not depend on efficient plasmid transformation and conjugation, and requires as few as one transformant or conjugated cell for transposon saturation mutagenesis. Using this new tool, we have generated a Tn5 transposon insertion library of P. aeruginosa PAO1 with a diversity of ~ 10 8 , which is ~ 2 logs larger than the best transposon insertion library of PAO1 and related Pseudomonas strains ever generated (Table 1 ). P. aeruginosa is an important opportunistic pathogen that frequently causes nosocomial infections and many of the strains are multidrug-resistant. The mutant PAO1 library we generated should also be valuable for P. aeruginosa pathogenesis studies.
To our knowledge, our plasmids are the only non- ts , conditional suicide plasmids used for transposon mutagenesis, and they replicate in a wide range of bacterial species [ 19 ]. In contrast, many ts mutant plasmids seem to have limited host ranges, either due to the limited host ranges of the parental plasmids, or due to the species-specificity of their ts phenotypes [ 10 , 11 , 14 , 15 , 16 , 17 , 36 ]. In addition, ts plasmids often require prolonged incubation at high temperatures for plasmid curing, which can be harsh conditions for bacterial growth and survival, thus may lead to accumulation of adaptive genetic changes. Additional file 8 : Table S1 is a detailed comparison of our systems (pSNC-mTn5 and pSNC-mTn5ME) with various ts plasmid-based platforms that have been used for transposon mutagenesis in Gram-negative bacterial species, which clearly shows that our systems will be more broadly useful. In addition to their utilities in transposon mutagenesis, the IPTG-controlled conditional suicide plasmids that we developed should have many other applications, such as for allelic exchange or as curable vectors for delivering gene targeting systems, e.g., TargeTrons, λ Red, RecET, etc [ 37 , 38 ].
In this work, we have developed a number of IPTG-controlled conditional suicide plasmids that contain the broad-host-range RSF1010 origin. Using one of the constructs to deliver a hyperactive mTn5 transposon, we showed that this system can be used for efficient mutagenesis of different bacterial species. As the assay condition is mild and the host range of the RSF1010 plasmid is extremely wide, we believe that our methodology will have broad applications in microbiology research.
Bacterial strains and growth conditions
E. coli DH10B was purchased from Invitrogen. A. baylyi (ATCC 33305) and P. aeruginosa PAO1 (ATCC BAA-47) were purchased from ATCC. Unless stated otherwise, all the strains were grown at 37 °C in Luria Broth (LB) liquid media with agitation at 200 rpm or on LB plates with 1.5% agar. For sacB counter selection, we used LBNS plates ( LB n o s alt: 1% Tryptone, 0.5% yeast extract and 1.5% agar) supplemented with 10% sucrose (Fisher Scientific). Appropriate antibiotics and concentrations were used to select for bacterial cells that are antibiotic resistant. E. coli DH10B: chloramphenicol (Cam; Gold Biotechnology), 25 μg/ml; kanamycin (Kan; Fisher Scientific), 50 μg/ml. A. baylyi : Cam, 10 μg/ml; Kan, 10 μg/ml. P. aeruginosa PAO1: Cam, 250 μg/ml; Kan, 500 μg/ml.
Plasmid construction
To construct plamsid pMMB- repF , we PCR-amplified the repF gene from pMMB208 [ 18 ]. The PCR fragment was digested with Hin dIII and Pst I, and inserted at the corresponding sites of pMMB208, downstream of the tac promoter (P tac ). To construct plasmid pMMB- repF / sacB , the sacB gene and its promoter were PCR amplified from plasmid pRE112 [ 22 ] and inserted between the unique Sac I and Kpn I sites of pMMB- repF .
To construct plasmids pMMB- repA K42A and pMMB- repA D139A, repA genes containing K42A and D139A mutations were generated in two-step PCRs from plasmid pMMB208 [ 21 ]. The mutant genes were cloned between the Hin dIII and Pst I sites of pMMB208.
Plasmid pSNC-mTn5 was constructed in multiple steps. First, plasmid pUT-mTn5Km/lacEZ was constructed from plasmid pUT-mTn5Km [ 4 ]. It contains a lac promoter-driven hyperactive transposase gene ( tnp H ) that has E54K, M56A and L372P mutations [ 3 ]. In addition, inside the mTn5 transposon, the inverted repeats flanking the kanamycin resistance marker ( Kan R ) were deleted [ 4 ]. The entire mTn5 cassette of pUT-mTn5Km/lacEZ, which contains the Kan R -mTn5 transposon and P lac - tnp H , was then PCR amplified and cloned at the Xba I site of pMMB- repF / sacB , resulting in plasmid pSNC-mTn5. It has an OE and an IE at the termini of the mTn5. Plasmid pSNC-mTn5ME was derived from pSNC-mTn5 by replacing both OE and IE with MEs.
Characterization of IPTG-induced plasmid loss and transposon mutagenesis
To test IPTG-induced plasmid loss of pMMB- repF , pMMB- repA K42A and pMMB- repA D139A, single colonies of E. coli DH10B cells transformed with the plasmids were inoculated into 5 ml LB + Cam media and cultured at 37 °C for ~ 14 h (h). After measuring OD 600 , 1 ml of each culture was pelleted by centrifugation and washed with 500 μl of fresh LB to remove antibiotics. Cells were then resuspended in 1 ml LB. An aliquot was added to 5 ml LB (final OD 600 = 0.001) with and without 1 mM IPTG and cultured at 37 °C for 24 h. 1 ml of the IPTG-induced samples was then pelleted, washed with 500 μl LB, and resuspended in 1 ml LB. Serial dilutions of the samples (±IPTG) were plated on LB and LB + Cam plates to evaluate plasmid loss. Plasmid retention frequencies were calculated as ratios of cfu (colony forming units) on LB + Cam plates and those on LB plates.
To perform transposon mutagenesis in E. coli DH10B, single colonies of pSNC-mTn5 and pSNC-mTn5ME transformants were cultured in 5 ml LB + Cam + Kan media overnight at 37 °C. Cells were then pelleted and washed as above to remove antibiotics, and an aliquot was inoculated to 5 ml LB (final OD 600 = 0.001) in a 14 ml culture tube and grown at 37 °C for 24 h with and without 1 mM IPTG induction. A 1 ml aliquot of the IPTG-induced samples was then pelleted, washed with 500 μl LBNS, and resuspended in 1 ml LBNS. Serial dilutions of the samples (±IPTG) were plated on LB and LB + Cam plates to evaluate plasmid loss. The IPTG induced samples were also plated on LBNS+ 10% sucrose and LBNS+ 10% sucrose+Cam plates to estimate percentage of plasmid-retaining cells in the presence of sucrose counter selection; and LBNS+ 10% sucrose+Kan plates to select for transposition events. Plasmid retention frequencies (PRF) were calculated as the following: (1) -IPTG: (cfu on LB + Cam)/(cfu on LB); (2) + IPTG: (cfu on LB + Cam)/(cfu on LB); (3) + IPTG+Suc: (cfu on LBNS+Suc + Cam)/(cfu on LBNS+Suc). Transposon retention frequencies (TRF) were calculated as the following: +IPTG+Suc: (cfu on LBNS+Suc + Kan)/(cfu on LBNS+Suc). mTn5 (or mTn5ME) transposition frequencies were calculated as TRF +IPTG + Suc – PRF +IPTG + Suc , which essentially equals to TRF +IPTG + Suc if the background (PRF +IPTG + Suc ) is low. The same protocol, except for the concentrations of antibiotics (indicated above) and IPTG (10 mM for PAO1), was followed to perform transposon mutagenesis in P. aeruginosa PAO1.
Similarly, to perform transposon mutagenesis in A. baylyi 33,305, single colonies of pSNC-mTn5 and pSNC-mTn5ME transformants were cultured in 5 ml LB + Cam + Kan media overnight at 37 °C. Cells were pelleted and washed as for E. coli and P. aeruginosa . Then, an aliquot was inoculated to 100 ml LB (final OD 600 = 0.001) in a baffled flask. The cultures were shaken vigorously (~ 250 rpm) at 37 °C for 24 h with and without 10 mM IPTG induction. A 1 ml aliquot of the IPTG-induced samples was then pelleted, washed with 500 μl LBNS, and resuspended in 1 ml LBNS. Serial dilutions of the samples (±IPTG) were then plated on appropriate plates to evaluate plasmid loss and mTn5 (or mTn5ME) transposition as in the assays for E. coli and for P. aeruginosa .
To verify plasmid loss in cells with potential transposition events, 100–150 Suc R Kan R colonies in each assay were then restreaked on LB + Kan and LB + Cam plates. In addition, presence of the transposon and the plasmid was determined by colony PCRs in a 25 μl reaction containing 25 mM TAPS-HCl (pH 9.3), 50 mM KCl, 2 mM MgCl 2 , 1 mM β-mercaptoethanol, 1x GC enhancer, 0.2 mM dNTPs, 0.1 μl of Q5 polymerase (2 u/μl; NEB), 1 μl of resuspended cells, and 150 ng each of the primers (final concentration = ~ 0.5 μM; see figure legends and in Additional file 9 : Table S2 form oligos used). PCRs were performed using the following condition: 1x (94 °C, 2 min); 25x (94 °C, 30 s; 50 °C, 30 s; 72 °C, 1 min); 1x (72 °C, 10 min); 1x (4 °C, hold).
Determination of transposon insertion sites
Transposon insertion sites in bacterial chromosomes were determined by arbitrarily primed PCR, in which transposon junctions were amplified in two steps [ 5 , 39 ]. Bacterial cells were resuspended in 10–20 μl of deionized water and 1 μl was used directly as the PCR template. In the first PCR step, the reaction was performed using a specific primer annealing to the transposon region (Tn5Km1) and a semi-degenerate primer (BDC1) that anneals to many sites on the bacterial chromosome. In the second step, aliquots of the first-round PCR products were amplified using a primer annealing to the transposon region (Tn5Km2), slightly closer to the insertion junction, and a non-degenerate primer (BDC2) that anneals to the constant region of the BDC1-derived sequence. PCRs were carried out under the conditions described above. PCR products from Step 2 were resolved in a 2% agarose gel and major products were gel-purified for sequencing to determine Tn5 insertion sites.
Construction of transposon insertion libraries of P. aeruginosa PAO1
To construct an mTn5 (or mTn5ME) insertion library of P. aeruginosa PAO1, plasmid pSNC-mTn5 (or pSNC-mTn5ME) was first electroporated into the bacterial cells. Ten transformants were cultured independently in 5 ml LB + Cam + Kan media at 37 °C for ~ 14 h. Equal amount of each sample (equivalent to 0.5 OD 600 × 1 ml) was then combined, pelleted, washed with 500 μl LB, and the pellet was resuspended in 1 ml LB. An aliquot of the mixture was then inoculated into 500 ml LB supplemented with 10 mM IPTG in a baffled flask (final OD 600 = 0.01) and shaken vigorously (300 rpm) at 37 °C for 24 h to perform transposon mutagenesis. The cells were then pelleted by centrifugation and washed with 250 ml LBNS medium. The pellet was resuspended in 50 ml LBNS medium and serial dilutions were plated on LB, LB + Cam, LBNS+ 10% sucrose, LBNS+ 10% sucrose+Cam, and LBNS+ 10% sucrose+Kan plates to determine plasmid loss, mTn5 (mTn5ME) transposition, and total library diversity. Arbitrary PCR and DNA sequencing were then performed to determine Tn5 insertion sites.
Determination of mTn5 and mTn5ME target site preferences in P. aeruginosa PAO1
To determine if mTn5 and mTn5ME have any target site preferences in P. aeruginosa PAO1, we generated sequence logos of their insertion sites in the bacterium using the WebLogo server ( https://weblogo.berkeley.edu/logo.cgi ). In total, 40 mTn5 insertion sites and 41 mTn5ME insertion sites were used for the analysis.
Abbreviations
Chloramphenicol
Isopropyl β-D-1-thiogalactopyranoside
Luria Broth
Optical density
Outside end
Temperature-sensitive
Phadnis SH, Berg DE. Identification of base pairs in the outside end of insertion sequence IS50 that are needed for IS50 and Tn5 transposition. Proc Natl Acad Sci U S A. 1987;84(24):9118–22.
Article CAS Google Scholar
Reznikoff WS. Transposon Tn5. Annu Rev Genet. 2008;42:269–86.
Goryshin IY, Reznikoff WS. Tn5 in vitro transposition. J Biol Chem. 1998;273(13):7367–74.
de Lorenzo V, Herrero M, Jakubzik U, Timmis KN. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol. 1990;172(11):6568–72.
Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, Will O, Kaul R, Raymond C, Levy R, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2003;100(24):14339–44.
Lee SA, Gallagher LA, Thongdee M, Staudinger BJ, Lippman S, Singh PK, Manoil C. General and condition-specific essential functions of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2015;112(16):5189–94.
Metzger M, Bellemann P, Schwartz T, Geider K. Site-directed and transposon-mediated mutagenesis with pfd-plasmids by electroporation of Erwinia amylovora and Escherichia coli cells. Nucleic Acids Res. 1992;20(9):2265–70.
Leahy JG, Jonesmeehan JM, Colwell RR. Transformation of Acinetobacter-Calcoaceticus Rag-1 by electroporation. Can J Microbiol. 1994;40(3):233–6.
Shan Z, Xu H, Shi X, Yu Y, Yao H, Zhang X, Bai Y, Gao C, Saris PE, Qiao M. Identification of two new genes involved in twitching motility in Pseudomonas aeruginosa. Microbiology. 2004;150(Pt 8):2653–61.
Sasakawa C, Yoshikawa M. A series of Tn5 variants with various drug-resistance markers and suicide vector for transposon mutagenesis. Gene. 1987;56(2–3):283–8.
CAS PubMed Google Scholar
Harayama S, Tsuda M, Iino T. Tn1 insertion mutagenesis in Escherichia coli K-12 using a temperature-sensitive mutant of plasmid RP4. Mol Gen Genet. 1981;184(1):52–5.
Le Breton Y, Mohapatra NP, Haldenwang WG. In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl Environ Microbiol. 2006;72(1):327–33.
Stubbendieck RM, Straight PD. Linearmycins activate a two-component signaling system involved in bacterial competition and biofilm morphology. J Bacteriol. 2017;199(18).
Maier TM, Pechous R, Casey M, Zahrt TC, Frank DW. In vivo Himar1-based transposon mutagenesis of Francisella tularensis. Appl Environ Microbiol. 2006;72(3):1878–85.
Rholl DA, Trunck LA, Schweizer HP. In vivo Himar1 transposon mutagenesis of Burkholderia pseudomallei. Appl Environ Microbiol. 2008;74(24):7529–35.
Pelicic V, Jackson M, Reyrat JM, Jacobs WR Jr, Gicquel B, Guilhot C. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1997;94(20):10955–60.
Choi KH, Mima T, Casart Y, Rholl D, Kumar A, Beacham IR, Schweizer HP. Genetic tools for select-agent-compliant manipulation of Burkholderia pseudomallei. Appl Environ Microbiol. 2008;74(4):1064–75.
Morales VM, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97(1):39–47.
Meyer R. Replication and conjugative mobilization of broad host-range IncQ plasmids. Plasmid. 2009;62(2):57–70.
Maeser S, Scholz P, Otto S, Scherzinger E. Gene F of plasmid RSF1010 codes for a low-molecular-weight repressor protein that autoregulates expression of the repAC operon. Nucleic Acids Res. 1990;18(21):6215–22.
Ziegelin G, Niedenzu T, Lurz R, Saenger W, Lanka E. Hexameric RSF1010 helicase RepA: the structural and functional importance of single amino acid residues. Nucleic Acids Res. 2003;31(20):5917–29.
Edwards RA, Keller LH, Schifferli DM. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene. 1998;207(2):149–57.
Barbe V, Vallenet D, Fonknechten N, Kreimeyer A, Oztas S, Labarre L, Cruveiller S, Robert C, Duprat S, Wincker P, et al. Unique features revealed by the genome sequence of Acinetobacter sp. ADP1, a versatile and naturally transformation competent bacterium. Nucleic Acids Res. 2004;32(19):5766–79.
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–64.
Wang N, Ozer EA, Mandel MJ, Hauser AR. Genome-wide identification of Acinetobacter baumannii genes necessary for persistence in the lung. MBio. 2014;5(3):e01163–14.
Article Google Scholar
Withers TR, Yin Y. Yu HD: identification of novel genes associated with alginate production in Pseudomonas aeruginosa using mini-himar1 mariner transposon-mediated mutagenesis. J Vis Exp. 2014;85:51346.
Google Scholar
Lewenza S, Falsafi RK, Winsor G, Gooderham WJ, McPhee JB, Brinkman FS, Hancock RE. Construction of a mini-Tn5-luxCDABE mutant library in Pseudomonas aeruginosa PAO1: a tool for identifying differentially regulated genes. Genome Res. 2005;15(4):583–9.
Gallagher LA, Shendure J, Manoil C. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. MBio. 2011;2(1):e00315–0.
Wong SM, Mekalanos JJ. Genetic footprinting with mariner-based transposition in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2000;97(18):10191–6.
Skurnik D, Roux D, Aschard H, Cattoir V, Yoder-Himes D, Lory S, Pier GB. A comprehensive analysis of in vitro and in vivo genetic fitness of Pseudomonas aeruginosa using high-throughput sequencing of transposon libraries. PLoS Pathog. 2013;9(9):e1003582.
Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A. 2006;103(8):2833–8.
Seet Q, Zhang LH. Anti-activator QslA defines the quorum sensing threshold and response in Pseudomonas aeruginosa. Mol Microbiol. 2011;80(4):951–65.
Klockgether J, Munder A, Neugebauer J, Davenport CF, Stanke F, Larbig KD, Heeb S, Schock U, Pohl TM, Wiehlmann L, et al. Genome diversity of Pseudomonas aeruginosa PAO1 laboratory strains. J Bacteriol. 2010;192(4):1113–21.
Green B, Bouchier C, Fairhead C, Craig NL, Cormack BP. Insertion site preference of mu, Tn5, and Tn7 transposons. Mob DNA. 2012;3(1):3.
Goryshin IY, Miller JA, Kil YV, Lanzov VA, Reznikoff WS. Tn5/IS50 target recognition. Proc Natl Acad Sci U S A. 1998;95(18):10716–21.
Maier TM, Havig A, Casey M, Nano FE, Frank DW, Zahrt TC. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl Environ Microbiol. 2004;70(12):7511–9.
Enyeart PJ, Mohr G, Ellington AD, Lambowitz AM. Biotechnological applications of mobile group II introns and their reverse transcriptases: gene targeting, RNA-seq, and non-coding RNA analysis. Mob DNA. 2014;5(1):2.
Court DL, Sawitzke JA, Thomason LC. Genetic engineering using homologous recombination. Annu Rev Genet. 2002;36:361–88.
Saavedra JT, Schwartzman JA, Gilmore MS. Mapping transposon insertions in bacterial genomes by arbitrarily primed PCR. Curr Protoc Mol Biol. 2017;118:15.15.11–5.
Download references
Acknowledgements
We thank Drs. Mark McIntosh, David Pintel and Donald H. Burke for helpful discussions.
This work was supported by the University of Missouri startup fund to H.G. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The dataset supporting the conclusion of this article are available from the corresponding author on reasonable request.
Author information
Santa S. Naorem and Jin Han contributed equally to this work.
Authors and Affiliations
Department of Molecular Microbiology and Immunology, University of Missouri School of Medicine, Columbia, MO, 65212, USA
Santa S. Naorem, Jin Han, Stephanie Y. Zhang, Junyi Zhang, Lindsey B. Graham, Angelou Song, Cameron V. Smith, Fariha Rashid & Huatao Guo
You can also search for this author in PubMed Google Scholar
Contributions
SSN, JH and HG conceived the study and designed the experiments; SSN, JH, SYZ, JZ, LBG, AS, CVS and FR performed the experiments; SSN and HG analyzed and wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Correspondence to Huatao Guo .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:.
Figure S1. Tn5 transposons. ( a ) Full-length Tn5. The full-length Tn5 contains two inverted IS50 elements at its ends. Only one of them encodes an active Tnp and an Inh (Inhibitor of Tnp). Kan R , kanamycin-resistance gene; Str R , streptomycin-resistance gene; and Ble R , bleomycin-resistance gene. ( b ) mTn5s. Top, an mTn5 with an OE and an IE at the termini. Bottom, an mTn5 with MEs at the ends. ( c ) Comparison of OE, IE and ME, with their polymorphisms highlighted in red. (TIF 4125 kb)
Additional file 2:
Figure S2. Confirmation of mTn5 transposition events in A. baylyi and in P. aeruginosa . ( a ) Colony restreaking assay of A. baylyi . 100 Suc R Kan R colonies of A. baylyi were restreaked on LB + Kan and LB + Cam plates, and all were found to be Kan R Cam S . 50 are shown here. ( b ) Colony PCR of 10 restreaked A. baylyi clones with the indicated primers. All were mTn5-positive and plasmid-negative. ( c ) Colony restreaking assay of P. aeruginosa . 100 Suc R Kan R colonies of P. aeruginosa were restreaked on LB + Kan and LB + Cam plates, and all were found to be Kan R Cam S . 50 are shown here. ( d ) Colony PCR of ten restreaked P. aeruginosa clones with primers indicated in the diagram. All were mTn5-positive and plasmid-negative. (TIF 6384 kb)
Additional file 3:
Figure S3. A transposon insertion library of P. aeruginosa PAO1 generated with pSNC-mTn5. ( a ) Plasmid and transposon retention frequencies of the mTn5 insertion library of P. aeruginosa PAO1. ( b ) Colony restreaking assay. 100 random Suc R Kan R colonies were restreaked on LB + Kan and LB + Cam plates. 100/100 were found to be Kan R Cam S and 50 are shown here. ( c ) Colony PCR of ten restreaked clones in ( b ). All were found to be mTn5-positive and plasmid-negative. ( d ) mTn5 insertion sites of 37 mutant clones from the transposon insertion library of P. aeruginosa . Identical clones are only shown once, and their numbers are indicated in parenthesis. (TIF 1363 kb)
Additional file 4:
Figure S4. Confirmation of mTn5ME transposition events in E. coli DH10B. ( a ) Colony restreaking. 100 random Suc R Kan R colonies of E. coli were restreaked on LB + Kan and LB + Cam plates. 100/100 were found to be Kan R Cam S and 50 restreaked colonies are shown here. ( b ) Colony PCR of ten restreaked clones in ( a ). All were found to be Tn5-positive and plasmid-negative. ( c ) Tn5 insertion sites of 13 independent DH10B clones. (TIF 7051 kb)
Additional file 5:
Figure S5. Confirmation of mTn5ME transposition events in A. baylyi 33,305. ( a ) Colony restreaking. 100 random Suc R Kan R colonies of A. baylyi were restreaked on LB + Kan and LB + Cam plates. 100/100 were found to be Kan R Cam S and 50 restreaked colonies are shown here. ( b ) Colony PCR of ten restreaked clones in ( a ). All were found to be Tn5-positive and plasmid-negative. ( c ) Sequence analysis shows that 9/14 A. baylyi clones had different Tn5 insertion sites. (TIF 6938 kb)
Additional file 6:
Figure S6. Confirmation of mTn5ME transposition events in P. aeruginosa PAO1. ( a ) Colony restreaking. 100 random Suc R Kan R colonies of P. aeruginosa PAO1 were restreaked on LB + Kan and LB + Cam plates. 100/100 were found to be Kan R Cam S and 50 restreaked colonies are shown here. ( b ) Colony PCR of ten restreaked clones in ( a ). All were found to be Tn5-positive and plasmid-negative. ( c ) Sequence analysis shows that 7/10 P. aeruginosa clones had different Tn5 insertion sites. (TIF 6280 kb)
Additional file 7:
Figure S7. Target site preferences of mTn5 and mTn5ME in P. aeruginosa . ( a ) Sequence logo of mTn5 insertion sites generated with WebLogo. 40 target sequences were analyzed. The 9 bp duplicated sequences adjacent to the OE are shown. There is a slight preference for certain nucleotides at several positions. ( b ) Sequence logo of mTn5ME insertion sites. 41 target sequences were analyzed. The 9 bp duplicated sequences adjacent to an ME are shown. It appears that mTn5ME has less nucleotide preference at the duplicated target sequence than mTn5 in Pseudomonas . (TIF 3834 kb)
Additional file 8:
Table S1. Comparison of conditional suicide vector-based transposon mutagenesis strategies used in Gram-negative bacteria. (TIF 7782 kb)
Additional file 9:
Table S2. List of primers used. (TIF 2042 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Naorem, S.S., Han, J., Zhang, S.Y. et al. Efficient transposon mutagenesis mediated by an IPTG-controlled conditional suicide plasmid. BMC Microbiol 18 , 158 (2018). https://doi.org/10.1186/s12866-018-1319-0
Download citation
Received : 12 July 2018
Accepted : 16 October 2018
Published : 24 October 2018
DOI : https://doi.org/10.1186/s12866-018-1319-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Transposon mutagenesis
- Conditional suicide plasmid
- Escherichia coli
- Pseudomonas
- Acinetobacter
BMC Microbiology
ISSN: 1471-2180
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 28 May 2013
Transposon insertion sequencing: a new tool for systems-level analysis of microorganisms
- Tim van Opijnen 1 &
- Andrew Camilli 2
Nature Reviews Microbiology volume 11 , pages 435–442 ( 2013 ) Cite this article
30k Accesses
338 Citations
28 Altmetric
Metrics details
Our knowledge of gene function has increasingly lagged behind gene discovery, hindering our understanding of the genetic basis of microbial phenotypes. Recently, however, massively parallel sequencing has been combined with traditional transposon mutagenesis in techniques referred to as transposon sequencing (Tn-seq), high-throughput insertion tracking by deep sequencing (HITS), insertion sequencing (INSeq) and transposon-directed insertion site sequencing (TraDIS), making it possible to identify putative gene functions in a high-throughput manner. Here, we describe the similarities and differences of these related techniques and discuss their application to the probing of gene function and higher-order genome organization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
A decade of advances in transposon-insertion sequencing
Technical considerations for cost-effective transposon directed insertion-site sequencing (TraDIS)
Long-read sequencing for identification of insertion sites in large transposon mutant libraries
Kleckner, N., Roth, J. & Botstein, D. Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J. Mol. Biol. 116 , 125–159 (1977).
Article CAS Google Scholar
Craig, N. L. Target site selection in transposition. Annu. Rev. Biochem. 66 , 437–474 (1997).
Kidwell, M. G. & Lisch, R. D. Transposable elements, parasitic DNA and genome evolution. Evolution 55 , 1–25 (2001).
Alekshun, M. N. & Levy, S. B. Molecular mechanisms of antibacterial multidrug resistance. Cell 128 , 1037–1050 (2007).
Kleckner, N., Chan, R. K., Tye, B.-K. & Botstein, D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J. Mol. Biol. 97 , 561–575 (1975).
Smith, V., Botstein, D. & Brown, P. O. Genetic footprinting: a genomic strategy for determining a gene's function given its sequence. Proc. Natl Acad. Sci. USA 92 , 6479–6483 (1995).
Smith, V., Chou, K. N., Lashkari, D., Botstein, D. & Brown, P. O. Functional analysis of the genes of yeast chromosome V by genetic footprinting. Science 274 , 2069–2074 (1996).
Akerley, B. J. et al. Systematic identification of essential genes by in vitro mariner mutagenesis. Proc. Natl Acad. Sci. USA 95 , 8927–8932 (1998).
Hensel, M. et al. Simultneous identification of bacterial virulence genes by negative selection. Science 269 , 400–403 (1995).
Mazurkiewicz, P., Tang, C. M., Boone, C. & Holden, D. W. Signature-tagged mutagenesis: barcoding mutants for genome-wide screens. Nature Rev. Genet. 7 , 929–939 (2006).
Mei, J. M., Nourbakhsh, F., Ford, C. W. & Holden, D. W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26 , 399–407 (1997).
Chiang, S. L. & Mekalanos, J. J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol. Microbiol. 27 , 797–805 (1998).
Jones, A. L., Knoll, K. M. & Rubens, C. E. Identification of Streptococcus agalactiae virulence genes in the neonatal rat sepsis model using signature-tagged mutagenesis. Mol. Microbiol. 37 , 1444–1455 (2000).
Autret, N., Dubail, I., Trieu-Cuot, P., Berche, P. & Charbit, A. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect. Immun. 69 , 2054–2065 (2001).
Lau, G. W. et al. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40 , 555–571 (2001).
Polissi, A. et al. Large-scale identification of virulence genes from Streptococcus pneumoniae . Infect. Immun. 66 , 5620–5629 (1998).
CAS PubMed PubMed Central Google Scholar
Hava, D. & Camilli, A. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45 , 1389–1406 (2002).
Loman, N. J. et al. High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity. Nature Rev. Microbiol. 10 , 599–606 (2012).
Bork, P. Powers and pitfalls in sequence analysis: the 70% hurdle. Genome Res. 10 , 398–400 (2000).
Galperin, M. Y. & Koonin, E. V. From complete genome sequence to 'complete' understanding? Trends Biotechnol. 28 , 398–406 (2010).
Kasif, S. & Steffen, M. Biochemical networks: the evolution of gene annotation. Nature Chem. Biol. 6 , 4–5 (2010).
Gawronski, J. D., Wong, S. M. S., Giannoukos, G., Ward, D. V. & Akerley, B. J. Tracking insertion mutants within libraries by deep sequencing and a genome-wide screen for Haemophilus genes required in the lung. Proc. Natl Acad. Sci. USA 106 , 16422–16427 (2009).
Langridge, G. C. et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 19 , 2308–2316 (2009).
Goodman, A. L. et al. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe 6 , 279–289 (2009).
van Opijnen, T., Bodi, K. L. & Camilli, A. Tn-seq: high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nature Methods 6 , 767–772 (2009).
van Opijnen, T. & Camilli, A. Genome-wide fitness and genetic interactions determined by Tn-seq, a high-throughput massively parallel sequencing method for microorganisms. Curr. Protoc. Microbiol. 19 , 1E.3.1–1E.3.16 (2010).
Google Scholar
Bentley, D. R. et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature 456 , 53–59 (2008).
Hernandez, D., François, P., Farinelli, L., Osterås, M. & Schrenzel, J. De novo bacterial genome sequencing: millions of very short reads assembled on a desktop computer. Genome Res. 18 , 802–809 (2008).
Holt, K. E. et al. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nature Genet. 40 , 987–993 (2008).
Nagalakshmi, U. et al. The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 320 , 1344–1349 (2008).
Liu, J. M. et al. Experimental discovery of sRNAs in Vibrio cholerae by direct cloning, 5S/tRNA depletion and parallel sequencing. Nucleic Acids Res. 37 , e46 (2009).
Article Google Scholar
Robertson, G. et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nature Methods 4 , 651–657 (2007).
Patwardhan, R. P. et al. High-resolution analysis of DNA regulatory elements by synthetic saturation mutagenesis. Nature Biotechnol. 27 , 1173–1175 (2009).
Ozsolak, F., Song, J. S., Liu, X. S. & Fisher, D. E. High-throughput mapping of the chromatin structure of human promoters. Nature Biotechnol. 25 , 244–248 (2007).
Alsford, S. et al. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res. 21 , 915–924 (2011).
Morgan, R. D., Dwinell, E. A., Bhatia, T. K., Lang, E. M. & Luyten, Y. A. The MmeI family: type II restriction–modification enzymes that employ single-strand modification for host protection. Nucleic Acids Res. 37 , 5208–5221 (2009).
Gallagher, L. A., Shendure, J. & Manoil, C. Genome-scale identification of resistance functions in Pseudomonas aeruginosa using Tn-seq. mBio 2 , e00315–10 (2011).
Griffin, J. E. et al. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 7 , e1002251 (2011).
Khatiwara, A. et al. Genome scanning for conditionally essential genes in Salmonella enterica serotype Typhimurium. Appl. Environ. Microbiol. 78 , 3098–3107 (2012).
Brutinel, E. D. & Gralnick, J. A. Anomalies of the anaerobic tricarboxylic acid cycle in Shewanella oneidensis revealed by Tn-seq. Mol. Microbiol. 86 , 273–283 (2012).
Klein, B. A. et al. Identification of essential genes of the periodontal pathogen Porphyromonas gingivalis . BMC Genomics 13 , 578 (2012).
Van Opijnen, T. & Camilli, A. A fine scale phenotype–genotype virulence map of a bacterial pathogen. Genome Res. 22 , 2541–2551 (2012).
Eckert, S. E. et al. Retrospective application of transposon-directed insertion site sequencing to a library of signature-tagged mini-Tn5Km2 mutants of Escherichia coli O157:H7 screened in cattle. J. Bacteriol. 193 , 1771–1776 (2011).
Dziva, F., van Diemen, P. M., Stevens, M. P., Smith, A. J. & Wallis, T. S. Identification of Escherichia coli O157: H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 150 , 3631–3645 (2004).
Crimmins, G. T. et al. Identification of MrtAB, an ABC transporter specifically required for Yersinia pseudotuberculosis to colonize the mesenteric lymph nodes. PLoS Pathog. 8 , e1002828 (2012).
Ooi, S., Shoemaker, D. & Boeke, J. DNA helicase gene interaction network defined using synthetic lethality analyzed by microarray. Nature Genet. 35 , 277–286 (2003).
Pan, X. et al. A DNA integrity network in the yeast Saccharomyces cerevisiae . Cell 124 , 1069–1081 (2006).
Parsons, A. B. et al. Integration of chemical-genetic and genetic interaction data links bioactive compounds to cellular target pathways. Nature Biotechnol. 22 , 62–69 (2004).
Fiedler, D. et al. Functional organization of the S. cerevisiae phosphorylation network. Cell 136 , 952–963 (2009).
Collins, S. et al. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446 , 806–810 (2007).
Tong, A. Global mapping of the yeast genetic interaction network. Science 303 , 808–813 (2004).
Schuldiner, M. et al. Exploration of the function and organization of the yeast early secretory pathway through an epistatic miniarray profile. Cell 123 , 507–519 (2005).
St Onge, R. P. et al. Systematic pathway analysis using high-resolution fitness profiling of combinatorial gene deletions. Nature Genet. 39 , 199–206 (2007).
Dixon, S., Costanzo, M., Baryshnikova, A., Andrews, B. & Boone, C. Systematic mapping of genetic interaction networks. Annu. Rev. Genet. 43 , 601–625 (2009).
Beltrao, P., Cagney, G. & Krogan, N. J. Quantitative genetic interactions reveal biological modularity. Cell 141 , 739–745 (2010).
Joshi, S. M. et al. Characterization of mycobacterial virulence genes through genetic interaction mapping. Proc. Natl Acad. Sci. USA 103 , 11760–11765 (2006).
Girgis, H., Liu, Y., Ryu, W. & Tavazoie, S. A. Comprehensive genetic characterization of bacterial motility. PLoS Genet. 3 , e154 (2007).
Christen, B. et al. The essential genome of a bacterium. Mol. Syst. Biol. 7 , 1–7 (2011).
Zhang, Y. J. et al. Global assessment of genomic regions required for growth in Mycobacterium tuberculosis . PLoS Pathog. 8 , e1002946 (2012).
Mann, B. et al. Control of virulence by small RNAs in Streptococcus pneumoniae . PLoS Pathog. 8 , e1002788 (2012).
Carette, J. E. et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science 326 , 1231–1235 (2009).
Papatheodorou, P. et al. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc. Natl Acad. Sci. USA 108 , 16422–16427 (2011).
Rosmarin, D. M. et al. Attachment of Chlamydia trachomatis L2 to host cells requires sulfation. Proc. Natl Acad. Sci. USA 109 , 10059–10064 (2012).
van Opijnen, T., Boerlijst, M. C. & Berkhout, B. Effects of random mutations in the human immunodeficiency virus type 1 transcriptional promoter on viral fitness in different host cell environments. J. Virol. 80 , 6678–6685 (2006).
Benjamin, W. H., Hall, P., Roberts, S. J. & Briles, D. E. The primary effect of the Ity locus is on the rate of growth of Salmonella typhimurium that are relatively protected from killing. J. Immunology 144 , 3143–3151 (1990).
CAS Google Scholar
Goodman, A., Wu, M. & Gordon, J. Identifying microbial fitness determinants by insertion sequencing using genome-wide transposon mutant libraries. Nature Protoc. 6 , 1969–1980 (2011).
Download references
Acknowledgements
T.v.O. was supported by a postdoctoral fellowship from the Netherlands Organization for Scientific Research (Rubicon-NWO) and the Charles H. Hood Foundation. A.C. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and affiliations.
Tim van Opijnen is at the Biology Department, Boston College, 140 Commonwealth Avenue, 420 Higgins Hall, Chestnut Hill, Massachusetts 02467, USA.,
Tim van Opijnen
Andrew Camilli is at the Howard Hughes Medical Institute and the Department of Molecular Biology and Microbiology, Tufts University School of Medicine, 136 Harrison Avenue, Boston, Massachusetts 02111, USA.,
Andrew Camilli
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Tim van Opijnen or Andrew Camilli .
Ethics declarations
Competing interests.
The authors declare no competing financial interests.
Related links
Further information.
Tim van Opijnen's homepage
Andrew Camilli's homepage
PowerPoint slides
Powerpoint slide for fig. 1, powerpoint slide for fig. 2.
A bacterial plasmid that contains a large eukaryotic DNA insertion (typically >150 kb) and can be used for cloning, genetic manipulation and transformation.
(Chromatin immunoprecipitation followed by sequencing). A method that uses crosslinking of a protein to DNA followed by immunoprecipitation of the complex and subsequent sequencing of the bound DNA to reveal the binding site of the protein.
Assays in which a wild-type copy of a gene is reintroduced into a cell or organism that lacks the gene. This can confirm that the phenotype is caused by disruption or deletion of the gene in question, and that this phenotype can be reversed.
Genes that once had a function, but through the accumulation of mutations, became inactive.
Methods that count the total number of reads obtained for a particular sequence after massively parallel sequencing (in contrast to hybridization-based methods of quantification).
A glass slide (or other surface) on which oligonucleotides or PCR products of defined sequence are spotted. These microarrays are used to quantify the nucleic acids within a sample by hybridization.
The inability of a single functional copy of a gene to produce a wild-type phenotype in a diploid organism. This occurs when the second copy of the gene is inactivated by mutation.
An analysis that indicates a putative function for a hypothetical virulence gene using fitness data for the gene obtained during growth in defined in vitro conditions.
Assays that measure the transcriptional activity of a gene promoter by converting the RNA transcripts to cDNAs and then using massively parallel sequencing to determine the number of cDNA molecules present.
A technique in which a transposon is tagged with a specific DNA sequence (a bar-code) that is used to determine the presence of the transposon in a DNA pool (as the amplified and labelled tag hybridizes to a probe on a membrane).
(Small-RNA sequencing).The discovery of non-coding sRNAs through direct sequencing of their cDNAs by massively parallel sequencing.
Experiments in which DNA is transferred from one bacterium to another by means of a bacteriophage.
An enzyme that cleaves DNA at a defined distance from an asymmetrical recognition site.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
van Opijnen, T., Camilli, A. Transposon insertion sequencing: a new tool for systems-level analysis of microorganisms. Nat Rev Microbiol 11 , 435–442 (2013). https://doi.org/10.1038/nrmicro3033
Download citation
Published : 28 May 2013
Issue Date : July 2013
DOI : https://doi.org/10.1038/nrmicro3033
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Mutualism reduces the severity of gene disruptions in predictable ways across microbial communities.
- Jonathan N V Martinson
- Jeremy M Chacón
- William R Harcombe
The ISME Journal (2023)

Precise cut-and-paste DNA insertion using engineered type V-K CRISPR-associated transposases
- Connor J. Tou
- Benjamin P. Kleinstiver
Nature Biotechnology (2023)
Identification of determinants for entering into a viable but nonculturable state in Vibrio alginolyticus by Tn-seq
- Jingxiao Cai
- Mengqing Zhou
Applied Microbiology and Biotechnology (2023)
Resolving Deleterious and Near-Neutral Effects Requires Different Pooled Fitness Assay Designs
- Anurag Limdi
- Michael Baym
Journal of Molecular Evolution (2023)
Genome-wide identification of genes required for alternative peptidoglycan cross-linking in Escherichia coli revealed unexpected impacts of β-lactams
- Henri Voedts
- Sean P. Kennedy
- Jean-Emmanuel Hugonnet
Nature Communications (2022)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Enabling low-cost and robust essentiality studies with high-throughput transposon mutagenesis (HTTM)
Antoine Champie
Amélie de grandmaison, simon jeanneau, frédéric grenier, pierre-étienne jacques, sébastien rodrigue.
- Author information
- Article notes
- Copyright and License information
Competing Interests: The authors have declared that no competing interests exist.
* E-mail: [email protected]
Received 2022 Nov 10; Accepted 2023 Mar 21; Collection date 2023.
This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Transposon-insertion sequencing (TIS) methods couple high density transposon mutagenesis with next-generation sequencing and are commonly used to identify essential or important genes in bacteria. However, this approach can be work-intensive and sometimes expensive depending on the selected protocol. The difficulty to process a high number of samples in parallel using standard TIS protocols often restricts the number of replicates that can be performed and limits the deployment of this technique to large-scale projects studying gene essentiality in various strains or growth conditions. Here, we report the development of a robust and inexpensive High-Throughput Transposon Mutagenesis (HTTM) protocol and validate the method using Escherichia coli strain BW25113, the parental strain of the KEIO collection. HTTM reliably provides high insertion densities with an average of one transposon every ≤20bp along with impressive reproducibility (Spearman correlation coefficients >0.94). A detailed protocol is available at protocol.io and a graphical version is also included with this article.
Introduction
The combination of transposon mutagenesis with next-generation sequencing revolutionized the study of gene essentiality [ 1 – 4 ]. This approach is based on the random integration of transposons within the genomes of a large population of cells that are next grown in selective conditions prior to transposon-insertion sequencing (TIS). Cells harboring deleterious insertions become less abundant or disappear from the population, revealing important or essential genes, while insertions persist in dispensable regions. By providing a high number of transposon insertions, TISallows a genome-wide evaluation of the importance of virtually any genomic feature. TIS has been adapted for a wide variety of cell types and was used to investigate the underlying genetic determinants of various phenotypes such as growth in specific media [ 2 ], motility [ 5 ], pathogenicity [ 6 ], as well as cell density [ 7 ].
Many TIS protocols have been described using different strategies for transposon delivery in bacterial cell populations [ 8 ], ranging from conjugative transfer of a suicide plasmid [ 9 ] to the electroporation of transposomes, which consist of purified transposases in complex with transposon DNA [ 10 ]. Several transposases have also been used, each with their specific characteristics including transposition efficiency, transposon recognition site identity, and target sequence biases [ 11 ]. Nevertheless, in most cases insertion site preferences are subtle and insertions occur essentially at random positions in the genome [ 12 ]. The widely used Tn 5 transposase, for instance, recognizes an optimized “mosaic” inverted repeat ( 5’-CTGTCTCTTATACACATCT-3’ ) [ 13 ] around the transposon. Which is then inserted with a slight preference for a 19 bp sequence with a more conserved 9 bp core 5′-G(CT)(CT)(CT)(AT)(AG)(AG)(AG)C-3′ [ 14 ]. This insertion bias is so light that in most cases the Tn 5 transposase is considered to insert transposons essentially at random [ 13 ]. Depending on the selected protocol and transposase, the complexity and cost of these approaches vary considerably but the number of samples that can be processed simultaneously remains usually low, mostly due to the lack of protocol optimization for high-throughput applications. Addressing this limitation would greatly facilitate large-scale or systematic gene essentiality projects.
We have thus developed a new high-throughput and inexpensive method for TIS. This “High-Throughput Transposon Mutagenesis” (HTTM) protocol is divided into three main steps: 1) mutagenesis, 2) DNA extraction, and 3) sequencing library preparation ( Fig 1 ). Each step has been optimized to obtain on average one transposon insertion per ≤20bp using Escherichia coli samples. A single person can process more than 960 samples per week without any specialized equipment. The total cost of the procedure per sample of a 96-well plate, from the initial bacterial culture inoculation to completed sequencing library preparation, is below 3$ and the hands-on time is approximately 4 minutes. HTTM not only shows an unprecedented capacity to handle numerous samples simultaneously, but also constitutes a robust, inexpensive, and time-effective alternative over conventional TIS methods [ 15 ] ( S2 File ).
Fig 1. Overview of the three main steps of the HTTM protocol.
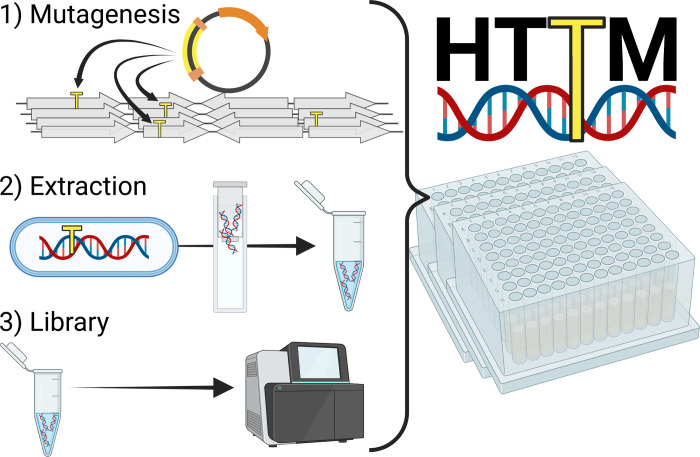
1) Transposons (yellow) are inserted at virtually random positions into the target genomes, creating a mutant pool. 2) Genomic DNA of the mutant pool is then extracted and 3) subjected to Illumina sequencing library preparation. All steps are conveniently performed in a 96-well plate format.
Mutagenesis
The HTTM mutagenesis step takes advantage of the E . coli MFD pir [ 16 ] strain as a conjugative transfer donor chassis strain containing the broad host range RP4 conjugative machinery integrated into its genome, a deletion of the dapA gene resulting in diaminopimelic acid auxotrophy, and a π replication protein cassette required for the proper replication of oriV R6K replicons. Importantly, the E . coli MFD pir strain is free of the Mu bacteriophage that could interfere with the analysis if inserted into the target strain’s genome along with the transposon [ 16 ]. A transposon mutagenesis plasmid, pFG051 ( Fig 2A ), was introduced in the MFD pir strain and contains an oriT RP4 sequence enabling its conjugative transfer into bacterial cells targeted for mutagenesis, a gene encoding a hyperactive Tn 5 transposase [ 17 ] under the control of a CI-repressed promoter, as well as a transposon conferring spectinomycin resistance. Finally, the repressor plasmid pAC017_cI was also introduced in MFD pir along with pFG051 to express the wild-type version of the λ bacteriophage cI repressor ( Fig 2B ). In contrast to the thermosensitive cI 857 variant commonly found in molecular biology laboratories worldwide, the wild-type CI repressor can fully repress its target promoter even at a temperature of 37°C. The resulting strain, eAC494, was used as the donor for every transposon mutagenesis experiment performed in this article. Since the expression of the transposase is tightly repressed in eAC494, early transposition events are prevented, ensuring that transposon insertions occur in the target bacterial population ( Fig 2C ). The target used in all experiments described in this article was an E . coli BW25113 derivative in which a neo (kanamycin resistance gene)- sgfp cassette was inserted into the lacZ gene. After conjugation, insertion mutants can be grown and sub-cultured in a selective medium to eliminate target cells with a transposon interrupting an essential gene or that simply did not receive a transposon, as well as the eAC494 donor strain which is subjected to diaminopimelic acid starvation.
Fig 2. HTTM relies on the conjugative delivery of a specialized transposon mutagenesis plasmid.
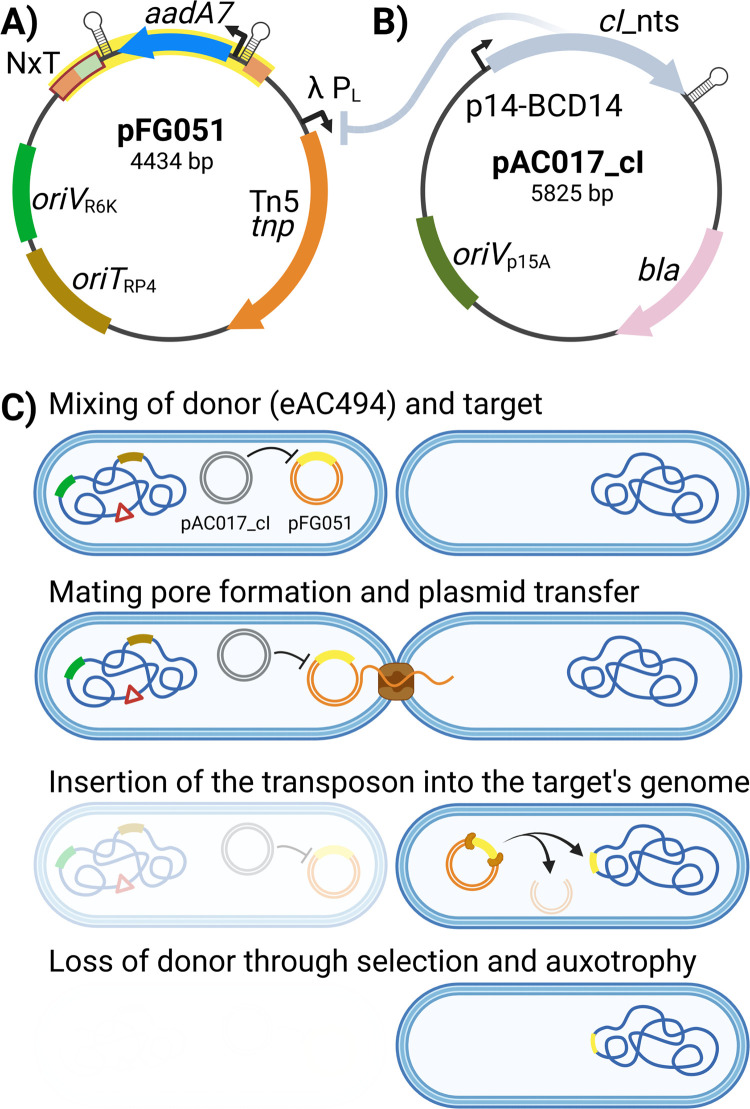
A) Map of the pFG051 suicide transposition plasmid. The transposon (yellow) consists of the aadA7 gene, conferring resistance to spectinomycin driven by a constitutive promoter, flanked by two Tn 5 inverted repeats (light orange boxes) and transcription terminators (stemloops). The transposon also contains an Illumina Nextera adapter sequence (light green) adjacent to one of the two Tn 5 inverted repeats, forming the complete Nextera adapter used in library preparation (NxT, red). pFG051 depends on the conditional R6K replication origin ( oriV R6K ) that is only active in the presence of the π protein exclusively expressed in the E . coli eAC494 donor strain. The origin of transfer ( oriT RP4 ) enables the mobilization of pFG051 by the broad host range RP4 conjugative machinery. The Tn 5 transposase is under the control of a wild-type CI λ repressor only present in the eAC494 donor strain. B) Plasmid pAC017_cI constitutively expresses the CI repressor in the eAC494 donor strain under the control of the p14-BCD14 promoter. pAC017_cI contains the bla gene conferring resistance to ampicillin and a low copy replication constitutive replication origin ( oriV p15A). cI_nts = cI _non thermo sensitive. C) Main steps involved in the conjugative delivery of the transposon mutagenesis plasmid pFG051. The donor strain (eAC494) contains genomic integration of the RP4 conjugative machinery (brown), the pir gene (dark green), and a dapA deletion (red triangle) as well as the λ cI repressor plasmid pAC017_cI (gray) and the transposon mutagenesis suicide plasmid pFG051 (yellow).
Bacterial conjugation could in principle be carried directly in broth using 96-well plates to easily increase the throughput of transposon mutagenesis. However, this approach has proven to be inefficient with the broad host range conjugative plasmid RP4 and would not provide enough mutants to reach high insertion densities ( Fig 3A ). In contrast, transfer rates are particularly high when the eAC494 and the target cells are combined on a solid support. To avoid the technical difficulties and costs associated with the deposition of conjugation mixture on cellulose filters or the inconvenient format of Petri dishes, molten agar medium was poured at the bottom of deep-well plates and allowed to solidify and dry for a few days under sterile conditions. Conjugative transfer on these “agar plugs” was then thoroughly optimized to consistently obtain ≥15 million mutants per well. Under optimized agar plug drying conditions, a conjugation mixture of 50 μl can be quickly absorbed by a dried 300 μl agar plug to generate the highest number of mutants ( Fig 3B ). However, this parameter may require optimization according to the exact model of deep-well plates and drying conditions. The impact of temperature on the number of insertion mutants was also evaluated, showing slightly higher yields at 37°C ( Fig 3C ). The donor-to-target (volume/volume) ratio was an important factor contributing to high conjugation rates. While a 10:1 ratio proved to be slightly more efficient at delivering the transposon, it also required the preparation of a higher volume of donor culture which could become limiting when performing very high-throughput experiments. Thus the 5:1 ratio was selected as it is more practical while keeping the efficiency very high ( Fig 3D ). The conjugation time was also investigated and a 2h period was observed to be a good compromise between method efficiency and required time, thus allowing to perform the mutagenesis step in a regular workday ( Fig 3E ). Once the conjugation has been performed, the cells can be easily resuspended using a thermomixer with the same efficiency as the more tedious manual up and down pipetting procedure ( Fig 3F ). The mutants are next subjected to several passages over a few days to select those that can still grow and survive under the desired selective conditions despite the transposon insertions. To avoid bottlenecks during our selective passages we optimized the volume of culture transferred each day aiming to preserve at least 3 million mutants at each passage. This estimation is based on the cell concentrations typically obtained with the targeted bacterial strain grown in the liquid medium used for mutant selection. The exact volume of culture transferred at each passage should be optimized according to the specific target or growth conditions. The original pre-selection library can optionally be saved for future use by simply pooling together and making a glycerol stock of the usually discarded passage 1.
Fig 3. Optimization of key HTTM parameters.
The total number of insertion mutants generated (blue) and the overall method efficiency (purple) were quantified while testing selected parameters such as A) the conjugation substrate (broth or agar plug), B) volume of conjugation mixture deposited on plug, C) conjugation temperature, D) the eAC494 donor to target cell ratio, and E) the duration of conjugative mating. F) Total number of colony forming units (CFU) recovered after the resuspension of the conjugative mixture using manual pipetting or the thermomixer procedure. The conditions selected for the standard HTTM protocol are indicated with an asterisk. Box and whiskers show mean and standard deviation across biological triplicates.
DNA extraction
Following transposon mutagenesis, the genomic DNA (gDNA) from the generated pool of mutants can be harvested after each passage to monitor insertion levels as a function of time or simply at a defined time point to evaluate essentiality after a specific number of generations. DNA extraction is performed using a commercially available 96-well array of silica columns. To reduce the cost and ecological footprint associated with this step, the columns are regenerated after each extraction using a previously described method [ 18 ]. Briefly, the columns are first washed using an alkaline & Triton X-100 solution, the remaining DNA is degraded with a concentrated acid & Triton X-100 solution before a final extensive wash of the columns using the same alkaline solution as well as water to remove any trace of residual DNA. The amount of DNA recovered was consistent over successive cycles of extraction and column regeneration while no carry-over DNA was detected (S1, S2 Figs in S6 File ). The recipes required for both DNA extraction and column regeneration solutions can be found in the online version of the protocol and the homemade versions were found to perform similarly to commercially available solutions (S3 Fig in S6 File ). Using the current protocol, the DNA extraction yield for all wells was found to consistently range from 1.5 to 2.5 μg. This makes the systematic quantification unnecessary before sequencing library preparation as these amounts are well above the minimal suggested input of 30 ng of genomic DNA, which corresponds to approximately 6 million transposon insertion mutant genomes.
Sequencing library preparation
Several sequencing platforms are available but given its widespread availability, the Illumina technology was selected for HTTM sequencing. The pFG051 plasmid was thus designed to contain an Illumina Nextera adapter sequence at one extremity of the transposon ( Fig 2A ), thereby positioning the start of sequencing reads exactly at the insertion site. This prevents any issue with cluster segregation due to the presence of identical sequences at the beginning of Illumina reads when reading through the transposon end and avoids dark cycles or the need to increase sequencing read diversity by spiking PhiX control DNA [ 19 ]. An additional advantage of this approach is the possibility of using shorter read lengths, which broadens options for kits and instruments that can be used.
The NEBNext Ultra II DNA Library Prep Kit for Illumina (NEB) was directly used for the gDNA fragmentation and ligation of adapters. However, the protocol was optimized to minimize reaction volumes, and purification was removed between most steps to reduce the time and cost related to library preparation. These changes did not affect the yield and specificity of the transposon amplification (S4 Fig in S6 File ) as long as the first PCR step contains a maximum of 2 μl of the unpurified ligation reaction in a 50 μl reaction (S5 Fig in S6 File ). Since 8 μl of adapter-ligated DNA is available after the ligation step, up to four PCR replicates can be made to maximize the number of insertion sites. The HTTM samples are then pooled and sequenced using an Illumina sequencing system. The resulting data is then subjected to conventional bioinformatics quality control steps that include the adapter and quality trimming, alignment, etc. Duplicated genes or sequences (e.g., rRNA genes or identical insertion elements) are generally excluded from the analysis since sequencing reads cannot be unambiguously mapped. Bio-Tradis [ 20 ] or other toolkits [ 21 , 22 ] then model the insertion densities across the genome into two distinct statistical populations, allowing the segregation of features likely essential from those that appear dispensable, respectively presenting low and high insertion densities (S6 Fig in S6 File ).
Material and methods
The protocols described in this peer-reviewed article is published on protocols.io:
Mutagenesis: DOI: dx.doi.org/10.17504/protocols.io.36wgq72n3vk5/v1
gDNA extraction: DOI: dx.doi.org/10.17504/protocols.io.q26g7yzz3gwz/v1
Library preparation: DOI: dx.doi.org/10.17504/protocols.io.n2bvj8oowgk5/v1
They are included for printing as Supporting Information S1 File with this article. The exhaustive list of reagents and consumables used in this protocol is described in S2 File .
To facilitate high-throughput supernatant removal from deep-well plates, a custom adaptor for vacuum pump, the Aspir-8, has been designed and 3D printed. Models and printing instructions can be found at: https://www.thingiverse.com/thing:5569608 . Aspir-8 allows rapid removal of all liquid in a 8-well row while preventing cross-contamination by using easily swappable p200 sterile tips. To further increase robustness and throughput, this adapter can be coupled with a guide that prevents pellet aspiration and leaves 50 μl of liquid at the bottom of the wells.
Expected results
The HTTM method was optimized and validated using a GFP-expressing E . coli BW25113 [ 23 ] derivative by performing 24 biological replicates. The conjugative delivery of the transposon on agar plugs consistently generates >15 million mutants ( S3 File ) that can next be grown over many passages under selective conditions. The gDNA from the mutant pools can then be extracted at the desired passage and the sequencing libraries prepared in 96-well plates. For E . coli grown in EZ Rich medium [ 24 ], 5 consecutive passages (~18 divisions) were found to provide well-defined transposon insertion profiles (S7 Fig in S6 File ) with an average of 261,309 ± 47,828 insertion sites per replicate at passage 5, corresponding to one insertion per ~16 bp ( Fig 4A ). These numbers are obtained by combining in silico the four PCR replicates obtained for each sample after the second PCR step of the Illumina library preparation protocol. Each supplementary PCR replicate adds a significant number of new sites but with diminishing returns as the number of replicates increases. On average, the number of different insertions raises by 61% by adding a second replicate, by 28% from a duplicate to a triplicate, and by 18% from a triplicate to a quadruplicate ( Fig 4B & S8 Fig in S6 File ). Interestingly, combining the four PCR replicates in silico and analyzing exactly 2 million reads per biological replicate would still provide 200,133 sites on average, representing 76% of the total insertions ( Fig 4C ). This suggests that a relatively low sequencing depth is usable under these HTTM conditions. The rate of non-unique transposon insertions in a single bacterium using high-efficiency delivery by bacterial conjugatation was previously estimated to be approximately 15% (25) but few studies report this information, making comparison difficult between the different methods.
Fig 4. Validation of the HTTM protocol in E . coli BW25113 using 24 biological replicates.
A) Number of transposon insertion sites detected in each replicate. Whiskers indicate 10–90 percentile. B) The average number of transposon insertion sites increases per additional PCR replicate included in the analysis. Whiskers indicate 10–90 percentile. C) Saturation curves showing the number of insertion sites as a function of the downsampling of each replicate. D) Spearman correlation coefficients of the gene insertion densities between the 24 replicates. E) Frequency of essential gene status attribution for each gene across the 24 replicates. F) Example of known essential genes (in red) at a representative locus showing the insertion sites (black lines) in each of the 24 replicates.
The reproducibility of the HTTM approach was next investigated by comparing the insertion density for each gene between the 24 replicates, showing a strong average Spearman correlation coefficient of 0.9425 ± 0.009 ( Fig 4D & S4 File ). The correlation coefficient stays almost identical when analyzing a subset of 2 million reads per biological sample (0.932 ± 0.007). Following gene essentiality calls in the E . coli BW25113 genome using the Bio-Tradis Toolkit [ 20 ], the different replicates were compared and 94% of all mappable genes have the same essentiality status across ≥23 samples (3742 consistently dispensable and 293 essential over the 4294 genes considered) ( Fig 4E & S5 File ). Interestingly, the remaining 6% have low insertion densities that are challenging to interpret for the Bio-Tradis toolkit and appear to be mostly composed of 1) genes that are very short (e.g., eyeA or ssb ; see S9 Fig in S6 File ) in which a single insertion could readily affect the essentiality status, or 2) gene interruptions leading to impaired fitness mutants that are not yet outcompeted by the rest of the population at passage 5 (e.g., the sapABCDF operon; see S10 Fig in S6 File ). The data can also be visualized on a genome browser to inspect the sequencing read distribution and investigate the essentiality of specific features ( Fig 4F ).
The high number of samples that can be processed in parallel by HTTM allows large-scale comparison of gene essentiality in different growth conditions or organisms [ 25 ]. The higher throughput facilitates the incorporation of several replicates in gene essentiality studies. In our experience, up to 960 samples can be processed by a single person every week with high-reproducibility and at a low cost (<3$ per sample). Since the RP4 conjugative machinery has a broad host range [ 26 – 28 ] only minor adjustments to the pFG051 plasmid and protocol fine-tuning would be necessary to apply this approach to other microorganisms. By allowing the investigation of gene essentiality under a wide diversity of conditions, we expect that HTTM will contribute to a better understanding of microbial cell functioning, propose a role for many unknown function genes, and facilitate the design of highly-engineered bacterial genomes.
Supporting information
Acknowledgments.
The authors are grateful to Dominick Matteau for his extensive review of the manuscript and Bruno Lemieux at the « Plateforme de purification des protéines » of the Université de Sherbrooke for the purification of the polymerase and the preparation of the Supermix 2X qPCR mix.
Associated content
Protocols on protocol.io DOI:
Aspir-8 3D model link :
https://www.thingiverse.com/thing:5569608
Data Availability
Raw Illumina sequencing data is available on SRA at the following address: https://www.ncbi.nlm.nih.gov/sra/PRJNA896618 .
Funding Statement
This research was enabled in part by support provided by the Centre de recherche du CHUS ( https://www.crchus.ca/en/home ) and by the Université de Sherbrooke ( https://www.usherbrooke.ca/ ) awarded to PEJ and SR. PEJ and SR both hold a Fonds de Recherche du Québec – Santé (FRQS) Research Scholar Career Award ( https://frq.gouv.qc.ca/sante/ ). The funders had and will not have a role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
- 1. van Opijnen T, Bodi KL, Camilli A. Tn-seq; high-throughput parallel sequencing for fitness and genetic interaction studies in microorganisms. Nat Methods. 2009. Oct;6(10):767–72. doi: 10.1038/nmeth.1377 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Langridge GC, Phan MD, Turner DJ, Perkins TT, Parts L, Haase J, et al. Simultaneous assay of every Salmonella Typhi gene using one million transposon mutants. Genome Res. 2009. Dec;19(12):2308–16. doi: 10.1101/gr.097097.109 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Goodman AL, McNulty NP, Zhao Y, Leip D, Mitra RD, Lozupone CA, et al. Identifying genetic determinants needed to establish a human gut symbiont in its habitat. Cell Host Microbe. 2009. Sep 17;6(3):279–89. doi: 10.1016/j.chom.2009.08.003 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. van Opijnen T, Levin HL. Transposon Insertion Sequencing, a Global Measure of Gene Function. Annu Rev Genet. 2020. Nov 23;54(1):337–65. doi: 10.1146/annurev-genet-112618-043838 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Kakkanat A, Phan MD, Lo AW, Beatson SA, Schembri MA. Novel genes associated with enhanced motility of Escherichia coli ST131. PLOS ONE. 2017. May 10;12(5):e0176290. doi: 10.1371/journal.pone.0176290 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Eichelberger KR, Sepúlveda VE, Ford J, Selitsky SR, Mieczkowski PA, Parker JS, et al. Tn-Seq Analysis Identifies Genes Important for Yersinia pestis Adherence during Primary Pneumonic Plague. mSphere. 2020. Aug 5;5(4):e00715–20. doi: 10.1128/mSphere.00715-20 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 7. Dorman MJ, Feltwell T, Goulding DA, Parkhill J, Short FL. The Capsule Regulatory Network of Klebsiella pneumoniae Defined by density-TraDISort. mBio. 2018. Nov 20;9(6):e01863–18. doi: 10.1128/mBio.01863-18 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Cain AK, Barquist L, Goodman AL, Paulsen IT, Parkhill J, van Opijnen T. A decade of advances in transposon-insertion sequencing. Nat Rev Genet. 2020. Sep;21(9):526–40. doi: 10.1038/s41576-020-0244-x [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Chiang SL, Rubin EJ. Construction of a mariner-based transposon for epitope-tagging and genomic targeting. Gene. 2002. Aug 21;296(1):179–85. doi: 10.1016/s0378-1119(02)00856-9 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Goodall ECA, Robinson A, Johnston IG, Jabbari S, Turner KA, Cunningham AF, et al. The Essential Genome of Escherichia coli K-12. mBio. 2018. Mar 7;9(1):e02096–17. doi: 10.1128/mBio.02096-17 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Green B, Bouchier C, Fairhead C, Craig N, Cormack B. Insertion site preference of Mu, Tn5, and Tn7 transposons. Mobile DNA. 2012. Feb 7;3:3. doi: 10.1186/1759-8753-3-3 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Chao MC, Abel S, Davis BM, Waldor MK. The Design and Analysis of Transposon-Insertion Sequencing Experiments. Nat Rev Microbiol. 2016. Feb;14(2):119–28. doi: 10.1038/nrmicro.2015.7 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Reznikoff WS. Transposon Tn 5 . Annual Review of Genetics. 2008. Dec;42(1):269–86. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Shevchenko Y, Bouffard GG, Butterfield YSN, Blakesley RW, Hartley JL, Young AC, et al. Systematic sequencing of cDNA clones using the transposon Tn5. Nucleic Acids Res. 2002. Jun 1;30(11):2469–77. doi: 10.1093/nar/30.11.2469 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Neil K, Allard N, Grenier F, Burrus V, Rodrigue S. Highly efficient gene transfer in the mouse gut microbiota is enabled by the Incl2 conjugative plasmid TP114. Commun Biol. 2020. Sep 22;3(1):523. doi: 10.1038/s42003-020-01253-0 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Ferrières L, Hémery G, Nham T, Guérout AM, Mazel D, Beloin C, et al. Silent Mischief: Bacteriophage Mu Insertions Contaminate Products of Escherichia coli Random Mutagenesis Performed Using Suicidal Transposon Delivery Plasmids Mobilized by Broad-Host-Range RP4 Conjugative Machinery. J Bacteriol. 2010. Dec 15;192(24):6418–27. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Martínez-García E, Calles B, Arévalo-Rodríguez M, de Lorenzo V. pBAM1: an all-synthetic genetic tool for analysis and construction of complex bacterial phenotypes. BMC Microbiol. 2011. Feb 22;11:38. doi: 10.1186/1471-2180-11-38 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Tagliavia M, Nicosia A, Gianguzza F. Complete decontamination and regeneration of DNA purification silica columns. Analytical Biochemistry. 2009. Feb 1;385(1):182–3. doi: 10.1016/j.ab.2008.10.021 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Ramsey KM, Ledvina HE, Tresko TM, Wandzilak JM, Tower CA, Tallo T, et al. Tn-Seq reveals hidden complexity in the utilization of host-derived glutathione in Francisella tularensis. PLoS Pathog. 2020. Jun 3;16(6):e1008566. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Barquist L, Mayho M, Cummins C, Cain AK, Boinett CJ, Page AJ, et al. The TraDIS toolkit: sequencing and analysis for dense transposon mutant libraries. Bioinformatics. 2016. Apr 1;32(7):1109–11. doi: 10.1093/bioinformatics/btw022 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Miravet-Verde S, Burgos R, Delgado J, Lluch-Senar M, Serrano L. FASTQINS and ANUBIS: two bioinformatic tools to explore facts and artifacts in transposon sequencing and essentiality studies. Nucleic Acids Res. 2020. Aug 19. doi: 10.1093/nar/gkaa679 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. DeJesus MA, Ambadipudi C, Baker R, Sassetti C, Ioerger TR. TRANSIT—A Software Tool for Himar1 TnSeq Analysis. PLoS Comput Biol [Internet]. 2015. Oct 8 [cited 2018 Nov 1];11(10). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4598096/ . doi: 10.1371/journal.pcbi.1004401 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000. Jun 6;97(12):6640–5. doi: 10.1073/pnas.120163297 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Neidhardt FC, Bloch PL, Smith DF. Culture Medium for Enterobacteria. J Bacteriol. 1974. Sep;119(3):736–47. doi: 10.1128/jb.119.3.736-747.1974 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Costanzo M, Baryshnikova A, Bellay J, Kim Y, Spear ED, Sevier CS, et al. The Genetic Landscape of a Cell. Science. 2010. Jan 22;327(5964):425–31. doi: 10.1126/science.1180823 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Simon R, Priefer U, Pühler A. A Broad Host Range Mobilization System for In Vivo Genetic Engineering: Transposon Mutagenesis in Gram Negative Bacteria. Nat Biotechnol. 1983. Nov;1(9):784–91. [ Google Scholar ]
- 27. Thomas CM. Incompatibility Group P Plasmids: Genetics, Evolution, and Use in Genetic Manipulation. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Samperio S, Guzmán-Herrador DL, May-Cuz R, Martín MC, Álvarez MA, Llosa M. Conjugative DNA Transfer From E. coli to Transformation-Resistant Lactobacilli. Frontiers in Microbiology [Internet]. 2021. [cited 2022 Dec 21];12. Available from: https://www.frontiersin.org/articles/10.3389/fmicb.2021.606629 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Decision Letter 0
Cinzia calvio.
19 Dec 2022
PONE-D-22-31056Enabling low-cost and robust essentiality studies with high-throughput transposon mutagenesis (HTTM)PLOS ONE
Dear Dr. Rodrigue,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
Please submit your revised manuscript by Feb 02 2023 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at [email protected] . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols . Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols .
We look forward to receiving your revised manuscript.
Kind regards,
Cinzia Calvio, PhD
Academic Editor
Journal Requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
https://journals.plos.org/plosone/s/file?id=ba62/PLOSOne_formatting_sample_title_authors_affiliations.pdf
2. We note that the grant information you provided in the ‘Funding Information’ and ‘Financial Disclosure’ sections do not match.
When you resubmit, please ensure that you provide the correct grant numbers for the awards you received for your study in the ‘Funding Information’ section.
3. In your Data Availability statement, you have not specified where the minimal data set underlying the results described in your manuscript can be found. PLOS defines a study's minimal data set as the underlying data used to reach the conclusions drawn in the manuscript and any additional data required to replicate the reported study findings in their entirety. All PLOS journals require that the minimal data set be made fully available. For more information about our data policy, please see http://journals.plos.org/plosone/s/data-availability .
Upon re-submitting your revised manuscript, please upload your study’s minimal underlying data set as either Supporting Information files or to a stable, public repository and include the relevant URLs, DOIs, or accession numbers within your revised cover letter. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories . Any potentially identifying patient information must be fully anonymized.
Important: If there are ethical or legal restrictions to sharing your data publicly, please explain these restrictions in detail. Please see our guidelines for more information on what we consider unacceptable restrictions to publicly sharing data: http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions . Note that it is not acceptable for the authors to be the sole named individuals responsible for ensuring data access.
We will update your Data Availability statement to reflect the information you provide in your cover letter.
4. We note that you have stated that you will provide repository information for your data at acceptance. Should your manuscript be accepted for publication, we will hold it until you provide the relevant accession numbers or DOIs necessary to access your data. If you wish to make changes to your Data Availability statement, please describe these changes in your cover letter and we will update your Data Availability statement to reflect the information you provide.
5. Please amend either the abstract on the online submission form (via Edit Submission) or the abstract in the manuscript so that they are identical.
6. Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Does the manuscript report a protocol which is of utility to the research community and adds value to the published literature?
Reviewer #1: Yes
Reviewer #2: Yes
2. Has the protocol been described in sufficient detail?
To answer this question, please click the link to protocols.io in the Materials and Methods section of the manuscript (if a link has been provided) or consult the step-by-step protocol in the Supporting Information files.
The step-by-step protocol should contain sufficient detail for another researcher to be able to reproduce all experiments and analyses.
3. Does the protocol describe a validated method?
The manuscript must demonstrate that the protocol achieves its intended purpose: either by containing appropriate validation data, or referencing at least one original research article in which the protocol was used to generate data.
4. If the manuscript contains new data, have the authors made this data fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #2: No
5. Is the article presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please highlight any specific errors that need correcting in the box below.
6. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: In this manuscript, the authors report an interesting method High-Throughput Transposon Mutagenesis (HTTM) protocol which is robust and inexpensive. HTTM reliably provides provides high insertion densities with impressive reproducibility. This article is important to the researchers in Transposon Insertion Sequencing field and will help progress the field forward. There are, however, several issues in the review that require the author's attention.
1、 Introduction.
“The combination of transposon mutagenesis with next-generation sequencing” should be described as “Transposon-insertion sequencing (TIS) methods” not “Tn-seq”.
Tn-seq based on the assembly of a saturated Mariner transposon (not Tn5) insertion library was presented by Tim van Opijnen and Henry L. Levin. Transposon sequencing (Tn-Seq), transposon-directed insertion site sequencing (TraDIS), insertion sequencing (INSeq) and high-throughput insertion tracking by deep sequencing (HITS) are four variations on the TIS method. Authors may refer to recent reviews by Amy K. Cain et al. and Tim van Opijnen et al., doi: 10.1038/s41576-020-0244-x, doi: 10.1146/annurev-genet-112618-043838.
2、 A transposon mutagenesis plasmid contains an oriTRP4 sequence enabling its conjugative transfer into bacterial cells targeted for mutagenesis to generate the huge number of mutants. The question is whether we can confirm that each bacterium carries only one single transposon insertion. We know, if a bacterium contains multiple gene mutations it may misestimate the fitness of gene after library selection. In Tn-seq technology, in vitro transposition and subsequent transformation are both low-frequency events, so it is believed that the overwhelming majority of transformants harbor single transposon insertions.
3、HTTM depends on the frequency of conjugative transfer of RP4 plasmid between bacteria, so can it be easy applied to other microbial species?
Reviewer #2: General comments:
Transposon mutagenesis coupled with next-generation sequencing (Tn-seq) has been a powerful high-throughput genetic screening method for functional genomics in bacteria. However, the generation of mutant libraries can be labour intensive and expensive. In this manuscript, the authors offer a complete protocol from making mutant library, DNA extraction to Illumina sequencing which is claimed to reduce cost and labour. While I have no major issues with the protocol and its feasibility, I do have several minor points that could benefit from further clarification.
1. Estimation of independent mutants: the authors stated that from each agar plug, they could obtain ~15 million mutants. I assume that this number was simple obtained by CFU count and a fair number of these CFU were siblings, leading to the “inflated” number of ~15 million mutants, yet when sequenced only showed ~261,309 insertion sites. If we assume that one insertion site represents one independent mutant, then the initial estimation of 15 million mutants is >50x over. Indeed, based on the statement in lines 230-231, I would interpret that I will need to pool 3-4 replicates to obtain a well-saturated mutant library. This should be clarified in the manuscript.
2. The use of Pearson correlation coefficient is sensitive to sample size and sample distribution. It is well-known (also shown in Fig S6) that insertion index/insertion density per gene is bimodal distributed while Pearson correlation works best for Gaussian distribution. I suggest that the authors use a more appropriate measurement for showing correlation. A supplementary figure of Bland-Altman plots might do.
3. The bias of mutant libraries: the mutagenesis protocol includes passaging for several days to select for the transposon mutants and remove the donors. However, these passages also adapt the transposon mutant population to this particular growth condition. The resulting mutant libraries are therefore not good starting libraries to be used to investigate a different challenge/growth condition. One might need to make new mutant libraries for each condition examined. This point should be discussed in the manuscript.
4. The link to raw data https://www.ncbi.nlm.nih.gov/sra/PRJNA896618 does not work (Error message: The following term was not found in SRA: PRJNA896618)
7. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
Reviewer #1: No
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/ . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at [email protected] . Please note that Supporting Information files do not need this step.
Author response to Decision Letter 0
Collection date 2023.
--We thank the reviewers for their precious comments and insights We appreciate the constructive remarks that strengthen our manuscript. The original comments are presented below in black. Our responses are shown in blue.
Reviewer #1: General comments:
In this manuscript, the authors report an interesting method High-Throughput Transposon Mutagenesis (HTTM) protocol which is robust and inexpensive. HTTM reliably provides high insertion densities with impressive reproducibility. This article is important to the researchers in Transposon Insertion Sequencing field and will help progress the field forward. There are, however, several issues in the review that require the author's attention.
1 Introduction.“The combination of transposon mutagenesis with next-generation sequencing” should be described as “Transposon-insertion sequencing (TIS) methods” not “Tn-seq”.Tn-seq based on the assembly of a saturated Mariner transposon (not Tn5) insertion library was presented by Tim van Opijnen and Henry L. Levin. Transposon sequencing (Tn-Seq), transposon-directed insertion site sequencing (TraDIS), insertion sequencing (INSeq) and high-throughput insertion tracking by deep sequencing (HITS) are four variations on the TIS method. Authors may refer to recent reviews by Amy K. Cain et al. and Tim van Opijnen et al., doi:10.1038/s41576-020-0244-x, doi: 10.1146/annurev-genet-112618-043838.
--The transposon mutagenesis nomenclature has been updated in the abstract and the introduction to use TIS instead of Tn-seq. The suggested review articles have also been cited. (see lines 49 and 69 of the track-change mode document)
2 A transposon mutagenesis plasmid contains an oriTRP4 sequence enabling its conjugative transfer into bacterial cells targeted for mutagenesis to generate the huge number of mutants. The question is whether we can confirm that each bacterium carries only one single transposon insertion. We know, if a bacterium contains multiple gene mutations it may misestimate the fitness of gene after library selection. In Tn-seq technology, in vitro transposition and subsequent transformation are both low-frequency events, so it is believed that the overwhelming majority of transformants harbor single transposon insertions.
--We expect our approach based on conjugation will likely have higher double transposition occurrence than other approaches that typically generate a lower number of mutants, which lower the chance of non-unique transposon insertion events. We previously tested a similar system based on the conjugation using the oriTRP4 and found that roughly 85% of all mutants (3/20 colonies tested) harboured a single transposon insertion ( https://savoirs.usherbrooke.ca/handle/11143/11637 ). We now cite this work at lines 270-273 of the revised manuscript to indicate the possibility that a cell contains more than one transposon. Recent studies in E. coli, for example https://doi.org/10.1128/mBio.02096-17 , using in vitro transposition do not disclose the rates of non-unique transposition events in a cell, making direct comparison difficult.
3 HTTM depends on the frequency of conjugative transfer of RP4 plasmid between bacteria, so can it be easy applied to other microbial species?
--The RP4 plasmid can transfer at relatively high rates in many organisms such as E. coli, Salmonella, Klebsiella, Pseudomonas, Vibrio, Acinetobacter, Xanthomonas, Rhizobium, Sphingomonas etc. For example, Gram-negative organisms like Acinetobacter and Sphingomonas ( https://doi.org/10.1111/j.1574-6968.1999.tb13543.x ), the RP4 conjugation system already displays a high transferfrequency (>1x10-2) suggesting that the HTTM system is already applicable. For Gram-positive organisms like Lactobaccilus casei, transfer rates (1.4x10-2) have already been observed () while in other like Staphylococcus aureus or eukaryotes much lower efficiencies have been reported: 2x10-8 to 5x10-5 ( https://doi.org/10.1111/j.1574-6968.1987.tb02558.x , https://doi.org/10.1128%2Fjb.180.24.6538-6543.1998 ) suggesting that another conjugative system may be required to carry the transposon or that the RP4 conjugative system requires an accelerated evolution ( https://doi.org/10.15252/msb.202110335 ) to perform efficiently towards those recipients. Overall, the system is likely to be already usable without any modification in a many organisms but will need to be adapted to perform adequately in others.
This point has been clarified in the conclusion (Lines 297-298)
Reviewer #2: General comments:
--The number of transposon insertion mutants is calculated by estimating colony forming units (CFU) immediately after the 2h conjugation between the eAC494 donor strain and the target bacteria. A fraction of the ~15 million mutants are certainly identical for at least two reasons:
1) The insertion of transposon can occur at the same site in two different cells, which would not be so surprising given that every transposon has a slight bias as discussed in the manuscript (see lines 77-83);
2) Some of the mutants may divide during the 2h conjugation period although these conditions are presumably not optimal for cell doubling;
However, the average of 261,309 insertions sites mentioned at lines 230-231 were obtained after 5 culture passages and do not represent the initial number of mutants. Only a subset of the initial mutants (approximately 9 million) obtained during conjugation are introduced in the initial passage and many mutants will be outcompeted and disappear from the population after a few generations or passages. It is therefore expected that the number of insertion sites observed at each passage will decrease through time.
The diversity of the initial library (immediately after conjugation) has not been estimated by sequencing, and the closest sequencing-based estimate we can provide is the number of different mutants present after 1 selective passage with approximately 440K mutants at a sequencing depth of 2 million reads.
The replicates mentioned on line 230-231 are technical replicates of a part of the library preparation protocol and are intended to extract more diversity from the same, already existing, pool of mutants while limiting potential PCR bias and are not related to the number of mutants present in the initial pool. This has been clarified in the at lines 24-265 of the revised manuscript (in track change mode).
--We thank the reviewer for this comment. We now present the average Spearman correlation coefficient for the 24 replicates in the text along with the complete data in the supplementary material (see Lines 57, 275, 277, and 288-289).
--The HTTM protocol as described in our manuscript is designed to have any growth condition investigated in parallel and avoids the step of stocking a library for future use. The stock performed after passage 4 is merely a safeguard in case of problem during the DNA extraction and is not intended to be used for future experiments. The generation of a reusable mutant library used to be part of an earlier version of the protocol and can be performed by simply pooling and cryopreserving the transposon mutagenesis leftover from each well instead of discarding it after the initial library growth. We revised the text to mention this possibility at line 193-194.
--SRA has been contacted and the raw data is now released.
Submitted filename: Rebutal Letter.docx
Decision Letter 1
21 Mar 2023
PONE-D-22-31056R1
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/ , click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at [email protected] .
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact [email protected] .
Additional Editor Comments (optional):
Reviewer #2: I thank the authors for incorporating my suggestions into their revised manuscript. I don't have any further comments.
Acceptance letter
31 Mar 2023
Dear Dr. Rodrigue:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact [email protected] .
If we can help with anything else, please email us at [email protected] .
Thank you for submitting your work to PLOS ONE and supporting open access.
PLOS ONE Editorial Office Staff
on behalf of
Dr Cinzia Calvio
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (2.5 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List
This is a preprint.
It has not yet been peer reviewed by a journal.
The National Library of Medicine is running a pilot to include preprints that result from research funded by NIH in PMC and PubMed.

Transposon mutagenesis libraries reveal novel molecular requirements during CRISPR RNA-guided DNA integration
Matt wg walker, sanne e klompe, dennis j zhang, samuel h sternberg.
- Author information
- Copyright and License information
Joint Authors
To whom correspondence should be addressed. Tel: +1-717-475-3658; [email protected] . Correspondence may also be addressed to Sanne E. Klompe. [email protected]
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License , which allows reusers to copy and distribute the material in any medium or format in unadapted form only, for noncommercial purposes only, and only so long as attribution is given to the creator.
CRISPR-associated transposons (CASTs) direct DNA integration downstream of target sites using the RNA-guided DNA binding activity of nuclease-deficient CRISPR-Cas systems. Transposition relies on several key protein-protein and protein-DNA interactions, but little is known about the explicit sequence requirements governing efficient transposon DNA integration activity. Here, we exploit pooled library screening and high-throughput sequencing to reveal novel sequence determinants during transposition by the Type I-F Vibrio cholerae CAST system. On the donor DNA, large mutagenic libraries identified core binding sites recognized by the TnsB transposase, as well as an additional conserved region that encoded a consensus binding site for integration host factor (IHF). Remarkably, we found that VchCAST requires IHF for efficient transposition, thus revealing a novel cellular factor involved in CRISPR-associated transpososome assembly. On the target DNA, we uncovered preferred sequence motifs at the integration site that explained previously observed heterogeneity with single-base pair resolution. Finally, we exploited our library data to design modified transposon variants that enable in-frame protein tagging. Collectively, our results provide new clues about the assembly and architecture of the paired-end complex formed between TnsB and the transposon DNA, and inform the design of custom payload sequences for genome engineering applications of CAST systems.
INTRODUCTION
Transposons are pervasive genetic elements capable of mobilizing between distinct genetic contexts using various targeting pathways, which serve as a potent force for genome evolution in all domains of life ( 1 – 3 ). Despite their abundance and sheer diversity, most transposons share a common feature in the presence of inverted repeat sequences that dictate the boundaries of the mobile element ( 4 ). These terminal transposon sequences are referred to as the left and right ends and typically encode one or more binding sites recognized by oligomeric transposase proteins through specific protein-DNA interactions.
DNA transposons (Class II) may be classified by their hallmark transposase domain, leading to major classes of DD(E/D) transposons, serine transposons, tyrosine transposons and Y1/Y2-transposons ( 5 ). DD(E/D)-family transposable elements encompass all known examples of “cut-and-paste” DNA transposons, which are excised from the donor site and inserted into the target site. This reaction relies on mechanistic and enzymatic symmetry, with transposase subunits executing identical chemical steps on both transposon ends that involve coordinated nucleophilic attacks during strand cleavage and joining ( 4 ). The transposase binding sites themselves, however, are not always positioned symmetrically. Whereas some transposons encode symmetrically-positioned transposase binding sites within otherwise identical left and right ends, including Tc1/mariner -family transposons such as Sleeping Beauty and Mos1 ( 6 , 7 ), other elements encode asymmetrically-positioned binding sites within distinct left and right ends, including Hermes , piggyBac , Bacteriophage Mu, P elements, and Tn 7 -family transposons ( 8 – 12 ). These asymmetric transposon ends often facilitate strict control over integration orientation, as in the well-characterized Tn 7 transposon ( 8 , 13 ), allowing one transposon end to be preferentially integrated adjacent to a target site, but the underlying mechanistic basis explaining this preference is unresolved.
Tn 7 -family transposons exhibit a modular nature in that they encode highly similar core transposition proteins — TnsA, TnsB, and TnsC — but distinct target site selection components. TnsB is a DDE-family integrase that recognizes and binds the transposon ends, catalyzes 3’ cleavage of both transferred strands at the donor site, and catalyzes the transesterification reaction at the target site. TnsA is an endonuclease protein that forms a heteromeric complex with TnsB and cleaves the 5’ end of the non-transferred strand, collectively allowing for full excision of the transposon from the donor site ( 14 , 15 ). TnsC is an AAA+ ATPase protein that communicates between the transposase module and the targeting component ( 16 , 17 ). Distinct Tn 7 -like transposons encode a sequence-specific DNA-binding protein as their targeting module, often of the TniQ family and referred to as TnsD, which directs transposition to a safe-harbor locus such as glmS , comM , yhiN and parE ( 18 – 23 ). In contrast, CRISPR-associated transposons (CASTs) use nuclease-deficient CRISPR-Cas systems to catalyze programmable, RNA-guided DNA transposition ( 19 , 24 – 28 ).
We previously reported RNA-guided transposition activity for VchCAST, a Tn 7 -like transposon from Vibrio cholerae (also referred to as Tn 6677 ), which mediates efficient and highly specific DNA integration in an E. coli heterologous host ( 24 ). VchCAST encodes a Type I-F CRISPR-Cas system that specifies integration sites through RNA-guided DNA targeting by a multi-subunit complex called Cascade ( 24 , 29 ). Importantly, Cascade binds DNA in complex with an accessory transposition protein, TniQ, which ultimately recruits TnsC and the heteromeric TnsAB transposase, in complex with the donor DNA, to assemble the catalytically active transpososome ( Figure 1A ) ( 29 , 30 ). DNA integration occurs roughly 50-bp downstream of the R-loop formed by TniQ-Cascade, but the sequence determinants underlying heterogeneous integration distances observed across distinct target sites remained enigmatic in our previous work ( 24 , 31 ). Additionally, the role and requirement for multiple putative TnsB binding sites within both transposon ends is unclear, limiting efforts to further engineer VchCAST as a DNA integration tool. Like other Tn 7 -family transposons, the transposon left and right ends exhibit distinct binding site patterns, and this asymmetric arrangement may also relate to the biased orientation with which transposon insertions occur ( 8 ).
Figure 1 |. Pooled library approach to investigate transposon end mutability.
( A ) Schematic of RNA-guided transposition with VchCAST. ( B ) Integration efficiency of the WT mini-transposon in both orientations when directed to a genomic lacZ target site, as measured by qPCR. ( C ) Number of transposon right and left end library variants tested in each category. ( D ) Pooled library transposition approach. Library members were synthesized as single-stranded oligos and cloned into a plasmid donor library (pDonor), with 8-bp barcodes (gray) located between the transposon end and cargo used to uniquely identify each variant. The donor library was used for transposition into the E. coli genome, and junction amplicons were generated to determine the representation of each library member within integrated products by NGS. ( E ) Schematic of the native VchCAST system from Vibrio cholerae (top), and relative T-RL integration activity for library members in which the left and right ends were sequentially mutagenized beginning internally (bottom). Each point represents the average activity from two transposition experiments using the same pooled donor library.
Here, we employ library-based experiments in combination with high-throughput sequencing to investigate DNA sequence requirements during RNA-guided transposition by VchCAST. By systematically mutating both transposon ends and measuring resulting DNA integration activity, we empirically identified predicted transposase binding sites and revealed sequence preferences that mediate transposase-transposon cognate specificity. Interestingly, our results indicated that the relative positioning of each transposase binding site plays a crucial role in defining the proper architecture of the transpososome complex, with spacing patterns that correspond to the helical pitch of double-stranded DNA. These mutational data also revealed the importance of an integration host factor (IHF) binding site within the left transposon end, and subsequent genetic knockout and rescue experiments confirmed the role of IHF in stimulating transposition efficiency in E. coli . Finally, we uncovered new sequence preferences at the site of integration, and we exploited our mutagenesis data to rationally engineer the transposon right end to enable in-frame tagging of endogenous protein-coding genes. Collectively, this work expands our understanding of both protein and DNA sequence requirements of Tn 7 -like transposons, reveals insights into the architecture of the transpososome complex, and provides new knowledge to inform the design of custom transposon sequences for genome engineering applications.
MATERIAL AND METHODS
Cloning, testing, and analysis of pooled pdonor libraries.
Donor plasmid (pDonor) libraries were generated by cloning transposon left or end variants into a donor plasmid, which was co-transformed with an effector plasmid (pEffector) that directed transposition into the E. coli genome (schematized in Figure 1D ). Each transposon end variant was associated with a unique 10-bp barcode that was used to uniquely identify variants in our sequencing approach, which relied on sequencing the starting plasmid libraries (input) and integrated products from genomic DNA (output) by NGS to determine the representation of each library member before and after transposition. To sequence the output, we independently amplified integration events in the T-RL and T-LR orientations using a cargo-specific primer flanking the transposon end and a genomic primer either upstream or downstream of the integration site. We wrote custom python scripts to compare each library member’s representation in the output to its representation in the input, allowing us to calculate the relative transposition efficiency of our custom transposon end variants.
To clone the transposon donor libraries, we first generated library variants as 200-nt single stranded pooled oligos (Twist Bioscience). 1 ng of oligoarray library DNA was PCR amplified for 12 cycles in 40 μL reactions using Q5 High-Fidelity DNA Polymerase (NEB) and primers specific to the right or left end library, in order to add restriction enzyme digestion sites. Amplicons were cleaned up and eluted in 45 μL mQ H 2 O (QIAquick PCR Purification Kit). As the backbone vector, we used a plasmid encoding a 775-bp mini-transposon, delineated by 147-bp of the native transposon left end and 75-bp of the native transposon right end, on a pUC57 backbone. The backbone vector and library insert amplicons were digested (AscI and SapI for the right end library, and NcoI and NotI for the left end library) at 37 °C for 1 h, gel purified, and ligated in 20 μL reactions with T4 DNA Ligase (NEB) at 25 °C for 30 min. Ligation reactions were cleaned up and eluted in 10 μL mQ H 2 O (MinElute PCR Purification Kit), and then used to transform electrocompetent NEB 10-beta cells in five individual electroporation reactions according to the manufacturer’s protocol. After recovery (37 °C for 1 h), transformed cells were plated on large 245 mm × 245 mm bioassay plates containing LB-agar with 100 μg/mL carbenicillin. Plates were scraped to collect cells, and plasmid DNA was isolated using the QIAGEN Plasmid Midi Kit.
Transposition experiments were performed in E. coli BL21(DE3) cells. pEffector encoded a CRISPR array (repeat-spacer-repeat), a native tniQ - cas8 - cas7 - cas6 operon, and a native tnsA-tnsB - tnsC operon, all under the control of a single T7 promoter on a pCDFDuet-1 backbone ( 31 ). 2 μL of DNA solution containing 200 ng of pDonor and pEffector in equal molar amount was used to co-transform electrocompetent cells according to the manufacturer’s protocol (Sigma-Aldrich). Four transformations were performed for each sample, and following recovery at 37 °C for 1 h, each transformation was plated on a large bioassay plate containing LB-agar with 100 μg/mL spectinomycin, 100 μg/mL carbenicillin, and 0.1 mM IPTG. Cells were grown at 37 °C for 18 h. Thousands of colonies were scraped from each plate, and genomic DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega).
Next-generation sequencing (NGS) amplicons were prepared by PCR amplification using Q5 High-Fidelity DNA Polymerase (NEB). 250 ng of template DNA was amplified in 15 cycles during the PCR1 step. PCR1 samples were diluted 20-fold and amplified in 10 cycles during the PCR2 step. PCR1 primer pairs contained one pDonor backbone-specific primer and one transposon-specific primer (input library), or one genomic target-specific primer and one transposon-specific primer (output library). PCR amplicons were resolved by 2% agarose gel electrophoresis and gel-purified (QIAGEN Gel Extraction Kit). Libraries were quantified by qPCR using the NEBNext Library Quant Kit (NEB). Sequencing for both input and output libraries were performed using a NextSeq Mid or High Output Kit with 150-cycles (Illumina). Additionally, the input libraries were also sequenced using a MiSeq with 300-cycles (Illumina).
NGS data analysis was performed using custom Python scripts. Demultiplexed reads were filtered to remove reads that did not contain a perfect match to the 19-bp primer binding sequence at the 3’-terminus of the transposon end. Then, the 10-bp sequence directly downstream of the primer binding sequence was extracted, which encodes a barcode that uniquely identifies each transposon end variant. The number of reads containing each library member barcode was counted. If a read did not contain a barcode that matched a library member barcode, it was discarded. The barcode counts were summed across two NGS runs using the same PCR2 samples for the input libraries. Two biologically independent replicates were performed for the output libraries. The relative abundance of each library member was then determined by dividing the barcode count of each library member by the total number of barcode counts. The fold-change between the output and input libraries was calculated by dividing the relative abundance of each library member in the output library by its relative abundance in the input library. This fold-change was then normalized by dividing the fold-change of each library member by the average fold-change of four wildtype library members that contained identical transposon ends but unique barcodes.
One source of experimental noise in our approach came from PCR recombination ( 32 ), in which barcodes became uncoupled from their associated transposon end variants during PCR amplification. We quantified the frequency of uncoupling by performing long-read Illumina sequencing (MiSeq, 250 cycles) to sequence both the barcode and full-length transposon end, and found that not all barcodes were coupled to their correct transposon end sequence ( Figure S1B ). However, uncoupled reads mapped to a diverse pool of sequences, with the most abundant incorrect sequence for each library member representing only a low percentage of total reads ( Figure S1C ). These data therefore indicate that uncoupling events did not largely affect the ability to calculate relative integration efficiencies for each library member.
Sequence logos were generated with WebLogo 3.7.4, and the VchCAST sequence logo in Figure 2B was generated from the six predicted TnsB binding sites.
Figure 2 |. Transposase binding site (TBS) requirements for VchCAST.
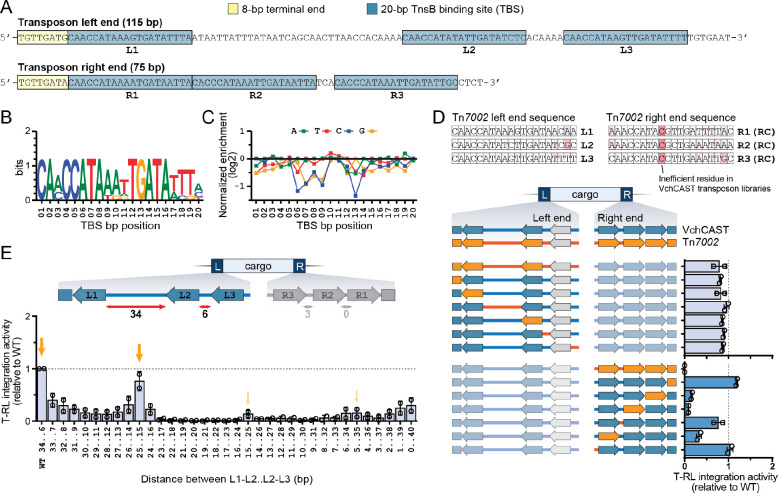
( A ) Schematic representation of the VchCAST transposon end sequences. Bioinformatically predicted transposase binding site (TBS) sequences are indicated with blue boxes and labeled L1-L3 and R1-R3. The 8-bp terminal end sequences that dictate the transposon boundaries are marked with yellow boxes. ( B ) WebLogo depicting the sequence conservation of the six bioinformatically predicted TBSs. ( C ) Relative integration efficiencies (log2-transformed) for mutagenized TBS sequences averaged over all six binding sites, shown as the mean for two biological replicates. ( D ) Top: Tn 7002 transposon end sequences are colored based VchCAST transposon end library data, where red indicates a relatively inefficient residue. Bottom: relative integration efficiencies of VchCAST/Tn 7002 chimeric ends verify critical compatibility sequence requirements of TBSs. Data are shown for two biological replicates. ( E ) Relative integration efficiencies for transposon variants containing altered distances between the indicated TBSs. Orange arrows highlight the 10-bp periodic pattern of activity. Data are shown for two biological replicates.
One limitation of our experimental setup is that we could not directly compare relative integration orientation within the same NGS libraries, since integration events were amplified independently in the T-RL and T-LR orientations. Instead, we inferred approximate integration efficiencies by comparing the enrichment scores of transposon end variants to those of wildtype variants within the same library. We also note that our strategy involved separate mutagenesis of either the left end or right end, but not both simultaneously. Finally, we stress that all transposition assays with pDonor libraries were performed heterologously in E. coli under overexpression conditions, and thus subtleties of transposon end recognition and binding that depend on regulated TnsB expression levels may be obscured.
Cloning, testing, and analysis of pooled pTarget libraries
pTarget libraries were designed to include an 8-bp degenerate sequence positioned 42-bp downstream of one of two potential target sites, as schematized in Figure 3B . Integration was directed to one of the two target sites flanking the degenerate sequence by a single plasmid (pSPIN) encoding both the donor molecule and transposition machinery under the control of a T7 promoter, on a pCDF backbone [described in ( 33 )]. To generate insert DNA for cloning the pTarget libraries, two partially overlapping oligos (oSL2241 and oSL2245, Table S2 ) were annealed by heating to 95 °C for 2 min and then cooling to room temperature. Annealed DNA was treated with DNA Polymerase I, Large (Klenow) Fragment (NEB) in 40 μL reactions and incubated at 37 °C for 30 min, then gel-purified (QIAGEN Gel Extraction Kit). Double-stranded insert DNA and vector backbone was digested with BamHI and AvrII (37 °C, 1 h); the digested insert was cleaned-up (MinElute PCR Purification Kit) and the digested backbone was gel-purified. Backbone and insert were ligated with T4 DNA Ligase (NEB), and ligation reactions were used to transform electrocompetent NEB 10-beta cells in four individual electroporation reactions according to the manufacturer’s protocol. After recovery (37 °C for 1 h), cells were plated on large bioassay plates containing LB-agar with 50 μg/mL kanamycin. Thousands of colonies were scraped from each plate, and plasmid DNA was isolated using the QIAGEN Plasmid Midi Kit. Plasmid DNA was further purified by mixing with Mag-Bind TotalPure NGS Beads (Omega) at a vol:vol ratio of 0.60 x and extracting the supernatant to remove contaminating fragments smaller than ~450 bp.
Figure 3 |. Transposase sequence preferences influence integration site patterns.
( A ) VchCAST exhibits target-specific heterogeneity in the distance (d) between the target site and integration site, which could result from sequence preferences within the downstream region (top). distances Deep sequencing revealed biases in integration site preference, with integration patterns shown for four target sites ( 4 – 7 ) located in the lac operon of the E. coli BL21(DE3) genome (top row) or encoded on a separate target plasmid (second row). Chimeric target plasmids that either maintain the 32-bp target site (third row) or 60-bp downstream region (bottom row) of target 4 were also tested. These data reveal that sequence identity of the downstream region (including the integration site), but not the target site, governs the observed in integration distance distribution. ( B ) Schematic of integration site library experiment, in which integration was directed into an 8-bp degenerate sequence encoded on a target plasmid (pTarget). ( C ) Sequence logo of preferred integration site, generated by selecting nucleotides from the top 5000 enriched sequences across all integration positions in each library, with a minimum threshold of four-fold enrichment in the integrated products compared to the input. ( D ) The preferred 5’-CWG-3’ motif in the center of the TSD is predictive of integration site distribution, as the displacement of this motif within the degenerate sequence shifts the preferred integration site distance, indicated by the red number.
2 μL of DNA solution containing 200 ng of pTarget and pSPIN at equal mass amounts were used to co-transform electrocompetent E. coli BL21(DE3) cells according to the manufacturer’s protocol (Sigma-Aldrich). Three transformations were performed and plated on large bioassay plates containing LB-agar with 100 μg/mL spectinomycin and 50 μg/mL kanamycin. Thousands of colonies were scraped from each plate, and plasmid DNA was isolated using the QIAGEN Plasmid Midi Kit.
Integration into pTarget yielded a larger plasmid than the starting input plasmid. To isolate the larger plasmid, we performed a digestion step that facilitated resolution of the integrated and unintegrated bands on an agarose gel, for extraction of the larger integrated plasmid. We performed this digestion step on both input and output libraries, digesting with NcoI-HF (37 °C for 1 h) and running them on a 0.7% agarose gel. The products were gel-purified (QIAGEN Gel Extraction Kit) and eluted in 15 μL EB in a MinElute Column (QIAGEN). 6.5 μL of cleaned-up DNA was used in each PCR1 amplification with Q5 High-Fidelity DNA Polymerase (NEB) for 15 cycles. PCR1 samples were diluted 20-fold and amplified in 10 cycles for PCR2. PCR1 primer pairs contained pTarget backbone-specific primers flanking a 45-bp region encompassing the degenerate sequence. Sequencing was performed with a paired-end run using a NextSeq High Output Kit with 150-cycles (Illumina).
NGS data analysis was performed using a custom Python script. Demultiplexed reads were filtered to remove reads that did not contain a perfect match to the 34- to 35-bp sequence upstream of the degenerate sequence for any i5-reads, or to the 45- to 46-bp sequence for any i7-reads. (35-bp and 46-bp was used for reads that were amplified from primers containing an additional nucleotide, which were used in PCR1 to generate cluster diversity during sequencing). For all reads that passed filtering, the 8-bp degenerate sequence was extracted and counted. The integration distance was determined in the output libraries by examining the i5 read sequence at an integration distance of 43-bp to 56-bp downstream of each target for the presence of the transposon right or left end sequence (20-nt of each end). The degenerate sequence was then extracted from either or both of the i5 and i7 reads, depending on the integration position. The degenerate sequence counts were summed across the two primer pairs. The relative abundance was determined by dividing the degenerate sequence count by the total number of degenerate sequence counts. Finally, the fold-change between the output and input libraries was calculated by dividing the relative abundance of each degenerate sequence at each integration position in the output library by its relative abundance in the input library, and then log2-transformed.
Sequence logos were generated with WebLogo 3.7.4. The preferred integration site logos in Figure S3A were generated from all degenerate sequences that were enriched four-fold in the integrated products compared to the input. The overall preferred integration site logos in Figure 3C and Figure S3D were generated by first applying the minimum threshold of four-fold enrichment in the integrated products compared to the input, and then selecting nucleotides from the top 5,000 enriched sequences across all integration positions. We selected nucleotides from the top 5,000 sequences from each library, yielding a total of 10,000 nucleotides at each position.
Endogenous gene tagging experiments
All VchCAST constructs were subcloned from pEffector and pDonor as described previously, using a combination of inverse (around-the-horn) PCR, Gibson assembly, restriction digestion-ligation, and ligation of hybridized oligonucleotides ( 24 , 31 ). pEffector encodes a CRISPR array (repeat-spacer-repeat), a native tniQ - cas8 - cas7 - cas6 operon, and a native tnsA-tnsB - tnsC operon, all under the control of a single T7 promoter on a pCDFDuet-1 backbone ( 31 ). Donor plasmids (pDonor) were designed to encode a mini-transposon (mini-Tn) with a wild-type 147-bp transposon left end and 57-bp linker-coding right end variant, on a pUC19 backbone. For endogenous gene tagging experiments, superfolder GFP (sfGFP) lacking a ribosome binding site (rbs) and start codon was cloned into the mini-Tn cargo region, and the mini-Tn was further cloned into a temperature-sensitive pSIM6 backbone.
Linker functionality constructs were designed to encode sfGFP with an extended 32-amino acid (aa) loop region between the 10 th and 11 th β-strands, under the control of a single T7 promoter, as described by Feng and colleagues ( 34 ). Linker variants encoding 18–19 aa were subcloned into the 32-aa loop region as follows. An entry vector was generated on a pCOLADuet-1 (pCOLA) vector harboring sfGFP, such that the 11th β-strand (GFP11) was replaced by the aforementioned extended 32-aa loop ( 34 ). Fragments encoding transposon right end linker variants and GFP11 were then amplified by conventional PCR and inserted into the extended loop region of the entry vector downstream of β-strands 1–10 (GFP1–10), such that total length of the loop remained constant at 32 aa.
To perform linker functionality assays, chemically competent E. coli BL21(DE3) cells were co-transformed with T7-controlled sfGFP linker functionality constructs (pCOLA) and an equal mass amount of empty pUC19 vector. Negative control transformants harbored either unfused sfGFP1–10 and sfGFP11 fragments on separate pCOLA and pUC19 backbones, respectively, or isolated sfGFP fragments. Transformed cells were plated on LB-agar plates with antibiotic selection (100 μg/mL carbenicillin, 50 μg/mL kanamycin), and single colonies were used to inoculate 200 μL of LB medium (100 μg/mL carbenicillin, 50 μg/mL kanamycin, 0.1 mM IPTG) in a 96-well optical-bottom plate. The optical density at 600 nm (OD600) was measured every 10 min, in parallel with the fluorescence signal for sfGFP, using a Synergy Neo2 microplate reader (Biotek) while shaking at 37 °C for 15 h. To derive normalized fluorescence intensities (NFI), all measured fluorescence intensities were divided by their corresponding OD600 values across all time points. A single representative NFI value was calculated per well by averaging all NFI values per well corresponding to OD600 values between 0.20 and 0.30, inclusive.
Transposition experiments were performed by transforming chemically competent E. coli BL21(DE3) cells harboring pEffector plasmids with pDonor plasmids by heat shock at 42 °C for 30 sec, followed by recovery in fresh LB medium. Recovery was performed at 30 °C for 1.5 h for temperature-sensitive pDonor plasmids, and 37 °C for 1 h for all other pDonor plasmids. Transformants were isolated on LB-agar plates containing the proper antibiotics and inducer (100 μg/mL carbenicillin, 50 μg/mL spectinomycin, 0.1 mM IPTG). After 43 h growth at 30 °C for temperature-sensitive pDonor plasmids, and 18 h growth at 37 °C for all other pDonor plasmids, samples were prepared for downstream qPCR analysis of integration efficiency or colony PCR identification of integration events.
For qPCR quantification, colonies were scraped from plates and resuspended in LB medium, and cell lysates were prepared for qPCR as described by Klompe and colleagues ( 24 ). Pairs of transposon- and target DNA-specific primers were designed to amplify fragments from integrated transposition products at the expected loci in either of two possible orientations. In parallel, a separate pair of genome-specific primers was designed to amplify an E. coli reference gene ( rssA ) for normalization purposes. qPCR reactions (10 μL) contained 5 μL of SsoAdvanced Universal SYBR Green Supermix (BioRad), 1 μL H2O, 2 μL of 2.5 μM primers, and 2 μL of hundredfold-diluted cell lysate and were prepared following transposition experiments as described above. Reactions were prepared in 384-well clear/white PCR plates (BioRad), and measurements were obtained in a CFX384 Real-Time PCR Detection System (BioRad). The following thermal cycling parameters were used: polymerase activation and DNA denaturation (98 °C for 3 min), and 35 cycles of amplification (98 °C for 10 s, 60 °C for 30 s). Each biological sample was analyzed in three parallel reactions: one reaction contained a primer pair for the E. coli reference gene, a second reaction contained a primer pair for one integration orientation, and a third reaction contained a primer pair for the other integration orientation. Transposition efficiency was calculated for each orientation as 2ΔCq, in which ΔCq is the Cq difference between the experimental and control reactions. Total transposition efficiency for a given experiment was calculated by summing transposition efficiencies across both orientations. All measurements presented were determined from three independent biological replicates.
For colony PCR identification of integration events, colonies were scraped from plates after transposition assays, resuspended in fresh LB medium, and re-streaked on LB-agar plates with the appropriate antibiotics and without IPTG inducer. To generate lysates, individual colonies were each transferred to 10 μL of H2O, followed by incubation at 95 °C for 2 min and centrifugation at 4,000 g for 5 min to pellet cell debris. Pairs of transposon- and target DNA-specific primers were designed to amplify fragments from integrated transposition products in the expected locus and orientation. In parallel, a separate pair of genome-specific primers was designed to amplify an E. coli reference gene ( rssA ) and determine whether the crude lysates were sufficiently dilute to allow successful amplification of the integrated transposition product. Transposition-less negative control samples were always analyzed in parallel with experimental samples to identify mispriming products that could result from the pDonor-containing crude lysates. PCR reactions (15 μL) contained 7.5 μL of 2X OneTaq 2X Master Mix with Standard Buffer (NEB), 5.9 μL H2O, 0.6 μL of 10 μM primers, and 1 μL of undiluted cell lysate as described above. PCR amplicons were resolved by 1% agarose gel electrophoresis and visualized by staining with SYBR Safe (Thermo Scientific). To verify in-frame integration events, amplicons of the expected length were excised after gel electrophoresis, isolated by the Gel Extraction Kit (Qiagen), and sent for Sanger sequencing (GENEWIZ).
Fluorescence microscopy experiments were performed as follows. A pEffector plasmid was designed to C-terminally tag the native E. coli msrB gene by integrating a mini-Tn encoding a linker variant (ORF2a) and sfGFP cargo in-frame with the coding sequence, thereby interrupting the endogenous stop codon. Transposition experiments were performed as described above by transforming chemically competent E. coli BL21(DE3) cells harboring pEffector plasmids with temperature-sensitive pDonor plasmids. Colonies were then scraped and resuspended in fresh LB medium. Resuspensions were diluted and re-streaked on double antibiotic LB-agar plates lacking IPTG (100 μg/mL carbenicillin, 50 μg/mL spectinomycin). After overnight growth on solid medium at 37 °C, individual colonies were used to inoculate liquid cultures (50 μg/mL spectinomycin) for overnight heat-curing at 37 °C, followed by replica plating on single and double antibiotic plates to isolate heat-cured samples. In tandem, colony PCR and Sanger sequencing (GENEWIZ) were performed to identify colonies with in-frame transposition products as described above. In preparation for fluorescence microscopy, Sanger-verified samples were inoculated in overnight 37 °C liquid cultures. On the day of imaging, 500 μL of saturated overnight cultures were transferred to 5 ml of fresh LB medium with the appropriate antibiotics. Aliquots of the newly inoculated cultures were removed around the stationary or mid-log phases and immobilized in glass slides coated with partially dehydrated aqueous 1% agarose-TAE pads. Immediately after immobilization, fluorescent microscopy was performed with a Nikon ECLIPSE 80i microscope using an oil immersion x100 objective lens, which was equipped with a Spot CCD camera and SpotAdvance software. All images were processed in ImageJ by normalizing background fluorescence.
Generating and testing E. coli knockout mutants
E. coli genomic knockouts of ihfA , ihfB , ycbG , hupA , hupB , hns , and fis were generated using Lambda Red recombineering, as previously described ( 35 ). Knockouts were designed to replace of each gene with a kanamycin resistance cassette, which was PCR amplified with Q5 High-Fidelity DNA Polymerase (NEB) using primers that contained 50-nt homology arms to knockout gene locus. PCR amplicons were resolved on a 1% agarose gel and gel-purified, eluting with 40 μL MQ (QIAGEN Gel Extraction Kit). Electrocompetent E. coli BL21(DE3) cells were prepared containing a temperature-sensitive plasmid that encodes the Lambda Red machinery under the control of a temperature-sensitive promoter (pSIM6). Protein expression from the temperature-sensitive promoter was induced by incubating cells at 42 °C for 25 min immediately prior to electrocompetent cell preparation. 300–600 ng of each insert was used to transform cells via electroporation (2 kV, 200 Ω, 25 μF), and cells were recovered overnight at 30 °C by shaking in 3 mL of SOC media. After recovery, 250 μL of culture was spread on 100 mm standard plates (LB-agar with 50 μg/mL kanamycin) and grown overnight at 30 °C. Kanamycin-resistant colonies were picked, and the genomic knock-in was confirmed by PCR amplification and Sanger sequencing using primer pairs flanking the knock-in locus.
VchCAST transposition experiments in E. coli knockout strains were performed by first preparing chemically competent WT and mutant cells and then transforming these strains with a single plasmid (pSPIN), which encodes the donor molecule and the native transposition machinery under the control of a T7 promoter and a crRNA targeting the lacZ genomic locus, on a pCDF backbone. After transformation by heat shock, cells were plated onto LB-agar with 100 μg/mL spectinomycin and 0.1 mM IPTG to induce protein expression, and incubated at 37 °C for 18 h. Hundreds of colonies were scraped from each plate, and integration efficiencies were quantified by the same qPCR assay described for the endogenous gene tagging experiments. Transposition experiments for other Type I-F homologs were performed as in the VchCAST experiments, except that the concentration of IPTG was reduced to 0.01 mM to mitigate toxicity.
Experiments that tested protein expression conditions in WT and ΔIHF cells were performed as described in the VchCAST transposition experiments. Promoters were varied from constitutive promoters (J23119, J23101) to inducible promoters (T7), for which different concentrations of IPTG were also tested.
For the complementation experiments, cells were co-transformed with pSPIN and a rescue plasmid (pRescue) that encoded both E. coli ihfA and ihfB under the control of separate T7 promoters on a pACYC backbone, and plated onto LB-agar with 100 μg/mL spectinomycin, 25 μg/mL chloramphenicol, and 0.1 mM IPTG to induce protein expression. Cells were incubated at 37 °C for 18 h, before colonies were scraped from each plate and integration efficiencies in both orientations were measured by qPCR.
To test DNA donor molecules with symmetric transposon ends, we cloned mutant pDonor encoding two right or two left transposon ends, and measured integration efficiency by co-transforming pDonor with pEffector under the control of a T7 promoter on a pCDF backbone. Cells were plated onto LB-agar with 100 μg/mL spectinomycin, 100 μg/mL carbenicillin, and 0.1 mM IPTG and incubated at 37 °C for 18 h, before colonies were scraped from each plate and integration efficiencies in both orientations were measured by qPCR.
EcoTn7 transposition experiments and NGS analysis.
To measure the integration efficiencies and distance distributions of EcoTn 7 in WT and E. coli mutant cells, we cloned genomic primer binding sites into the mini-Tn cargo of a single plasmid for Tn 7 transposition, which encoded a native tnsA-tnsB-tnsC-tnsD operon under the control of a constitutive pJ23119 promoter, on a pCDF backbone. The genomic primer binding sites were cloned adjacent to the transposon left and right ends such that the NGS amplicon length would be the same for unintegrated products and integrated products in either orientation (schematized in Figure S7A ). To quantify integration efficiencies using qPCR, we used primer pairs designed to amplify integrated products in both orientations, with one primer adjacent to the right transposon end a second primer either upstream or downstream of the integration site.
To quantify integration efficiencies by NGS, we amplified genomic DNA using a single primer pair with one primer complementary to the genomic primer binding site and the second primer complementary to the 3’-end of the glmS locus. Genomic DNA was extracted using the Wizard Genomic DNA Purification Kit (Promega). 250 ng of genomic was used in each PCR1 amplification with Q5 High-Fidelity DNA Polymerase (NEB) for 15 cycles. PCR1 samples were diluted 20-fold and amplified in 10 cycles for PCR2. Sequencing was performed with a paired-end run using a NextSeq High Output Kit with 150-cycles (Illumina).
NGS data analysis was performed using a custom Python script. Demultiplexed reads were filtered to remove reads that did not contain a perfect match to the first 65-bp of expected sequence resulting from either non-integrated genomic products or from integration events spanning 0-bp to 30-bp downstream of the glmS locus, and then counted the number of reads matching each of these possible products.
A pooled library approach to investigate transposon end sequence requirements
We set out to systematically mutagenize the transposon left and right end sequences of V. cholerae Tn 6677 using large pooled oligoarray libraries, building off our previous study of the VchCAST system ( 24 ). Starting with a minimal pDonor design that directed efficient genomic integration in both of two possible orientations ( Figure 1B ), we designed thousands of variants of the left (L) and right (R) end sequences, including truncations, base-pair substitutions, and transposase binding site modifications ( Figure 1C and Table S3 and S4 ). We assigned each variant a unique 8-bp barcode located between the mutagenized transposon end and the cargo, obviating the requirement to sequence across the entire transposon end to identify each variant. Each library also included four wildtype (WT) variants associated with unique barcodes, which we used to approximate the relative integration efficiency of each mutagenized library member. Libraries were then synthesized as single-stranded oligos, cloned into a mini-transposon donor (pDonor), and carefully characterized using next-generation sequencing (NGS), which demonstrated that all members were represented in the input sample for both transposon left and right end libraries ( Figure S1A – D ).
We performed transposition experiments by transforming E. coli BL21(DE3) cells expressing the transposition machinery with pDonor encoding either the left end or right end library, amplifying successful genomic integration products in both orientations via junction PCR ( Figure 1D ), and subjecting PCR products to NGS analysis. An enrichment score was then calculated for each variant, revealing a wide range of integration efficiencies, with most library members exhibiting diminished integration relative to the four WT samples ( Figure S1D ). Finally, we used enrichment scores of the WT library members for normalization, yielding a score for each variant that represented its relative activity. To validate our approach, we performed two biological replicates for each library transposition experiment and found strong concordance between both datasets, especially in the dominant T-RL integration orientation ( Figure S1E ). Importantly, we also rigorously determined the background level of library member–barcode uncoupling, given the high degree of sequence similarity between library members, which established contributors of experimental noise in our datasets ( Figure S1B – C and Methods).
The strength of the pooled-library approach is apparent by examining the effect of one category of variations, in which we sequentially mutated the transposon end sequences starting 120-nt into the transposon end, effectively creating end truncations, albeit without a change in overall mini-transposon size ( Figure 1E ). These results revealed the minimal transposon end sequence length: in the left end, ~105 bp were required for efficient integration, corresponding to all three predicted transposase (TnsB) binding sites, whereas in the right end, only ~50 bp were required, corresponding to the first two transposase binding sites. These findings are consistent with previous literature and adds single-bp resolution to the minimal transposon end sequences needed for efficient integration ( 24 ).
Transposase activity depends on specific sequence requirements
TnsB is integral to the mobilization of Tn 7 -like transposons, in that it catalyzes the excision and integration chemistry while also conferring sequence specificity for the transposon ends through recognition of repetitive sequence elements known as TnsB binding sites (TBSs) ( 8 , 15 , 36 ). Sequence analysis of the native VchCAST ends revealed three conserved TBSs in both the left and right ends ( Figure 2A , B and Figure S2A ) ( 24 ), and we verified these sequence requirements by examining a mutational panel at single-bp resolution ( Figure 2C and Figure S2B ). This dataset revealed that individual TBS point mutations can affect efficiency, particularly for positions 1, 6–9, and 12–14, but are not critical for integration. This more lenient sequence requirement is in line with recently published cryo-EM structures of DNA-bound TnsB from Tn 7 and Type V-K CAST systems, which revealed that many protein-DNA interactions occur with the phosphodiester backbone rather than specific nucleobases ( 37 – 39 ).
Experiments with E. coli Tn 7 showed that the internal TBSs are occupied before the more terminal sites ( 8 ). Even though the six TBSs of VchCAST differ by only a couple bases, we wondered if these differences might be biologically important, by enforcing a specific assembly pathway. To test this hypothesis, we tested all possible combinations of TBSs for the left and right ends, which we defined as L1–L3 and R1–R3 ( Figure S2C ). For both VchCAST ends, site 1 displayed the greatest TBS preference and preferred the L1/L3/R1 sequence, whereas site 2 preferred L1/R1/R2 and site 3 exhibited the least TBS preference but favored L3. We observed a preference for R1 in the first position on the left end, and a preference for L1 in the first position on the right end, suggesting that transposition might be favored when the terminal end sequences are identical (whether based on equal affinity or otherwise).
Apart from regulating transposition frequency, TBS sequence identity could also explain the propensity of a given CAST system to cross-react with related transposon substrates ( 18 ). We previously showed that VchCAST could efficiently mobilize mini-transposon substrates from three homologous CAST systems, but not Tn 7002 . To determine which Tn 7002 sequences were incompatible with mobilization by VchCAST machinery, we designed chimeric transposon ends that contain parts of both the VchCAST and Tn 7002 transposon ends ( Figure 2D ). The data revealed that chimeric left ends allowed for near WT integration efficiencies whereas chimeric right ends drastically decreased integration efficiency, likely due to the deleterious presence of a cytidine at position 9 of R1–R3 ( Figure 2D ). These data thus demonstrate that TBS sequence identity imparts specific constraints on the substrate recognition of a transposase for its cognate transposon DNA.
Finally, we sought to investigate the conserved positioning of TBSs within the transposon ends, after hypothesizing that the specific distance between TBSs might facilitate proper assembly of transposase subunits within a paired-end-complex (PEC) ( 18 ). After testing a mutagenic panel in which the length between TBSs was systematically varied ( Figure 2E and Figure S2D ), we found that even single-bp perturbations caused drastic changes in integration efficiency. Additionally, we detected an intriguing pattern of increasing and decreasing integration efficiencies at roughly 10-bp intervals, suggesting that the three-dimensional positioning of transposase proteins on helical DNA is important for transposition.
Together, these data highlight the impact of TBS mutations and TBS sequence positioning on transposition, and provide clues about how TBSs may have evolved to direct efficient assembly of synaptic paired-end complexes.
Transposase sequence preferences influence integration site patterns
In our previous work, we showed that VchCAST integration patterns differed in subtle but reproducible ways between distinct genomic target sites ( 24 , 31 ). Since integration is the result of both RNA-guided DNA targeting and transposase-mediated DNA integration, we were curious to investigate which DNA sequences and protein machineries were responsible for the heterogeneity in integration products. We first compared integration site patterns for four endogenous E. coli target sequences, designated 4–7, either at their native genomic location or on an ectopic target plasmid by deep sequencing ( Figure 3A ). Integration site patterns were notably distinct between the four targets but were highly consistent between genomic and plasmid contexts, suggesting that these patterns are dependent on local sequence alone and independent of other factors such as DNA replication or local transcription. Next, to disentangle contributions of the 32-bp target sequence (complementary to crRNA guide) from the downstream region including the integration site, we tested target plasmids that contained chimeras of the four target regions ( Figure 3A ). Remarkably, integration patterns for these chimeric substrates closely mirrored the patterns observed for the non-chimeric substrates when the ‘downstream region’ was kept constant, clearly indicating that the 32-bp target sequence does not modulate selection of the integration site.
We hypothesized that, like other transposases, TnsB might exhibit local sequence preferences immediately at the site of DNA insertion, and that these preferences could explain the observed heterogeneity in integration site patterns ( 40 ). To test this possibility, we generated a target plasmid (pTarget) library encoding two target sequences flanking an 8-bp degenerate sequence, such that integration events directed by a crRNA matching either target would lead to insertion directly into the degenerate 8-mer sequence ( Figure 3B ). We sequenced the target plasmids before and after transposition and compared the representation of integration site sequences to determine which sequences were enriched after transposition. These analyses revealed striking nucleotide preferences at conserved positions relative to the integration site ( Figure 3C and Figure S3A ). Specifically, there were clear biases for a YWR motif within the central three nucleotides of the target-site duplication (TSD), as well as a preference for D (A, T, or G) and H (A, T, or C) at the −3 and +3 positions relative to the TSD, respectively. Similar TSD preferences were previously observed for the Type V-K ShCAST system ( 25 ), suggesting that they may be broadly applicable to TnsB-family transposases.
To further explore the deterministic role of the preferred motif within the TSD, we plotted the distribution of reads containing a central 5’-CWG-3’ motif at different positions within the degenerate sequence. We focused on this motif because it favored a more unimodal distribution for the integration site by avoiding a centrally-preferred A or T nucleotide flanking the W. We found that this motif was indeed predictive of the preferred integration site distance that was sampled by VchCAST ( Figure 3D ). We extended this observation by plotting the distribution of reads containing multiple 5’-CWG-3’ motifs within the integration site and found that two copies of this preferred motif within the integration site conferred a bimodal distribution, wherein there were not one but two preferred integration sites within the degenerate sequence ( Figure S3B ). Finally, we leveraged our library data to predict the integration site distribution of previously targeted locations ( 24 ) and found that we could explain their differences at single-bp resolution ( Figure S3E ).
Both of the two distinct crRNAs and corresponding target sites on pTarget yielded consistent sequence preferences for both the TSD and +/− 3-bp positions ( Figure S3A ), but we were surprised to find that the preferred integration distance was shifted by 1 bp when comparing the two ( Figure S3C ). We suspected that this difference could be due to sequences preferences at the +/− 3-bp position that fell outside the degenerate sequence, and indeed, when we examined the sequences flanking the 8-mer library, we found that the downstream target (target B) contained a disfavored nucleotide in the −3-bp position for insertions that would occur with the 49-bp distance ( Figure S3D ). Interestingly, the role for these positions in modulating transposition behavior is well-substantiated by a recent structure of TnsB from Scytonema hofmanni Type V-K CAST bound to strand-transfer intermediates ( 41 ), which showed residue K290 of both terminal TnsB protomers contacting the +/− 3-bp position of the target site.
Role of boundary sequences and right end internal features on DNA integration
We next focused our attention on additional sequence features at the outermost edges of mini-transposon substrates. VchCAST and many other Tn 7 -like transposons encode an 8-bp terminal end immediately adjacent to the first transposase binding site, with the terminal TG dinucleotide highly conserved among a broad spectrum of transposons including IS3, Tn 7 , Mu and even retrotransposons ( 43 – 46 ). Integration data with library variants that featured mutations within these terminal residues revealed that positions 1–3, but not 4–8, were critical for efficient transposition ( Figure S4B ). This result is consistent with the DNA-bound cryo-EM structure of TnsB from a Type V-K CAST system, in which base-specific interactions were observed for the terminal TG dinucleotide ( 37 ), and with experiments indicating that these terminal dinucleotides are important for the formation of a stable Mu transpososome complex ( 44 , 47 ). Sequences beyond the terminal TG are also acted upon during excision of Tn 7 -like transposons, since the endonuclease TnsA cleaves the 5’ ends of the donor DNA 3 bp outside the transposon end boundaries ( 42 ). This observation suggested the possibility that the sequence context of the transposon donor itself might play a role in efficient transposition. However, library variants with mutations in the 5-bp sequence flanking the mini-transposon were integrated with equivalent efficiencies ( Figure S4A ), indicating that transposition machinery does not exhibit sequence specificity within this region.
To investigate whether the spacing between the terminal TG dinucleotide and the first TBS mattered, we tested variants that modulated the distance between the 8-bp terminal end and TBS1 ( Figure S4C ). Adding a single base pair in either the left or right end still allowed for efficient transposition, whereas transposition was completely ablated with the removal of 1 bp or addition of 2 bp, indicating tight control over this spacing. Interestingly, larger bp additions or deletions between the TG dinucleotide and first TBS were in some cases also permitted, but always with a concomitant shift in the transposon boundary that was actually mobilized and integrated at the target site ( Figure S4C ); in all cases, transposition still required a terminal TG. These data therefore suggest that the critical feature within the terminal end sequence is the TG dinucleotide, and that the ~8-bp spacing between this dinucleotide and the first TBS is a critical constraint for efficient transposition.
We also further investigated the importance of a palindromic sequence found 97–107 bp from the transposon right end boundary. Previous work suggested that this sequence might affect integration orientation, possibly by promoting transcription of the tnsABC operon, which would be consistent with empirical expression data and the AT-richness of the transposon end ( 48 ). To test this possibility, we mutated the palindromic sequence and found that variants with this sequence shifted the orientation preference towards T-LR, with just one arm of the palindrome (P B ) being sufficient to shift the orientation bias ( Figure S4D – E ). We also included bona fide constitutive promoters in place of the palindromic sequence and found that promoters directing transcription inwards (towards the cargo) did not impact integration orientation, whereas promoters directed outwards (across the right end) shifted the orientation preference towards T-LR, perhaps by antagonizing stable assembly of TnsB selectively at the right end ( Figure S4F ). These data highlight the role of this right end sequence region on integration orientation, which should be considered when designing custom cargo sequences.
Endogenous protein tagging with rationally engineered right ends
The left and right end sequences are critical for transposon DNA recognition and excision/integration, and transposition products therefore necessarily include these sequences as ‘scars’ at the site of insertion. We sought to exploit this feature and use our new knowledge of the mutability of the transposon ends to convert these scars into functional sequences that encode amino acid linkers for downstream protein tagging applications. We focused on the shorter right end, starting with a minimal 57-bp sequence, and observed that stop codons were present in all three possible open reading frames (ORF) for the WT sequence ( Figure 4A ) ( 24 ). When we tested a library of rationally designed right end variants that replaced stop codons and codons encoding bulky and/or charged amino acids, we identified numerous candidates for each possible ORF that maintained near-wild-type integration efficiency ( Figure S5A ). After validating library data by testing individual linker variants for genomic integration in E. coli ( Figure 4B ), we next set up a fluorescence-based assay to test for functionality of the encoded amino acid linkers.
Figure 4 |. Engineered transposon right ends enable functional in-frame protein tagging.
( A ) An illustration of the minimal transposon right end sequence (“WT-min.”) and the amino acids it encodes in three different reading frames. The 8-bp terminal end (yellow box) and TBSs (blue boxes) are shown. ( B ) Integration efficiencies for individual pDonor variants in which stop codons and codons encoding bulky/charged amino acids were replaced, as determined by qPCR. “Vector only” refers to the negative control condition where pEffector was co-transformed with a vector that did not encode a transposon. ( C ) Select right end linker variants were cloned in between the 10 th and 11th β-strands of GFP, in order to identify stable polypeptide linkers that still allow for proper formation and fluorescence activity of GFP. Normalized fluorescence intensity (NFI) was calculated using the optical density of each culture and is plotted for each linker variant alongside wildtype GFP. ( D ) Schematic of a proof-of-concept experiment in which the endogenous E. coli gene msrB is tagged by targeted, site-specific RNA-guided transposition (top). Fluorescence microscopy images reveal functional tagging of MsrB with the linker variant right end, but not the WT, stop codon-containing right end (bottom). Scale bar represents 10 μm.
GFP naturally consists of eleven β-strands that are connected by small loop regions, and a prior study demonstrated that the loop region between the 10 th and 11 th β-strand can be extended with novel linker sequences while still allowing for proper folding and fluorescence of the variant GFP protein ( 34 ). We cloned selected transposon right end variants into the loop region between β-strand 10 and 11 and measured GFP fluorescence intensity after expression of each construct, which revealed a subset of variants that were fully functional ( Figure 4C and Figure S5B ). Next, we selected the endogenous E. coli gene msrB for C-terminal tagging in a proof-of-concept experiment ( Figure 4D ). After generating a pDonor construct that encodes a right end linker variant with an adjacent, in-frame GFP gene lacking a promoter or start codon, we performed transposition experiments and used Sanger sequencing to verify that integration interrupted the endogenous stop codon while placing the linker and GFP sequence directly in-frame. Finally, proper expression of MsrB-GFP fusion proteins was analyzed by analyzing cells via fluorescence microscopy that received either the WT transposon right end or the linker variant, demonstrating that only the modified right end variant elicited the expected cellular fluorescence ( Figure 4D and Figure S5C ). Together, these data provide the basis for new genome engineering tools that allow for facile, endogenous gene tagging with single-bp control.
Integration Host Factor (IHF) binds the left transposon end to stimulate transposition
Closer inspection of the transposon left end mutational data revealed a sequence between the two terminal TnsB binding sites (TBSs) that, when mutated, led to reproducible transposition defects ( Figure 5A ). We noticed that the corresponding DNA sequence perfectly matched a consensus binding sequence for Integration Host Factor (IHF) ( 49 , 50 ), a heterodimeric nucleoid-associated protein (NAP) that binds to the consensus sequence 5’-WATCARNNNNTTR-3’ and induces a DNA bend of more than 160° ( 51 ). First identified as a host factor for bacteriophage λ integration, IHF is also involved in diverse cellular activities including chromosome replication initiation, transcriptional regulation, and various site-specific recombination pathways ( 52 – 54 ). This observation suggested the intriguing possibility that IHF might also play a role in RNA-guided transposition by CAST systems.
Figure 5 |. IHF involvement in RNA-guided transposition by VchCAST.
( A ) Library mutagenesis data for the transposon left end. Each point represents the effect of 4-bp mutations, averaged across 4 variants per base. ( B ) Integration activity of VchCAST in WT, Δ ihfA , and Δ ihfB cells. Integration activity was rescued by a plasmid encoding both ihfA and ihfB (pRescue). Each point represents integration efficiency measured by qPCR for one independent biological replicate. ( C ) Integration activity when the IHF binding site (IBS) is mutated (Mut), in which all consensus bases within the IBS were modified (from 5’-AATCAGCAAACTTA-3’ to 5’CCGACTCAACGGC-3’). ( D ) Conservation of the IBS in the transposon left end of twenty Type I-F CAST systems [first described in Klompe et al., 2022] ( E ) Sequence logo generated by aligning the left end sequence of all homologs around the conserved IHF binding site. ( F ) Integration activity in WT and ΔIHF cells for five highly active Type I-F CAST systems. Asterisks indicate the degree of statistical significance:* p ≤ 0.05, ** p ≤ 0.01, ***p ≤ 0.001. ( G ) Model: IHF binds the left end to resolve the spacing between the first two TBSs, bringing together TnsB protomers to form an active transpososome.
To test whether the IHF binding site (IBS) in the left transposon end functions to promote transposition, we first generated IHF knockout strains by mutating either ihfA and ihfB , and then measured integration efficiency with WT VchCAST. Deletion of either ihfA or ihfB decreased integration efficiency in the mutant strains by ~20-fold ( Figure 5B ), and this effect was completely rescued when we introduced a plasmid encoding recombinant ihfA or ihfB , confirming the IHF knockouts as causative genetic perturbations ( Figure 5B ). Interestingly, the reduction in integration efficiency was sensitive to vector design and expression conditions, as integration was less dependent on IHF when the donor DNA was encoded on a separate plasmid from the transposition machinery compared to when the donor DNA was encoded on the same plasmid as the transposition machinery ( Figure S6A ). When we selectively mutated the conserved IBS residues of a transposon donor, we found that transposition with the mutant left end decreased integration efficiency in WT cells, but not ΔIHF cells ( Figure 5C ). These experiments indicate that the IBS within the left transposon end is bound by IHF, and that IHF plays a role in stimulating RNA-guided transposition.
We next wondered whether the IHF requirement was conserved across diverse I-F CAST systems, taking advantage of the twenty homologous systems that we recently described ( 18 ). Visual examination of the transposon left ends revealed a highly conserved IBS across all homologs ( Figure 5D , E ), and aligning the sequence between the first two TBSs using Clustal Omega also revealed the IBS consensus as a conserved feature ( Figure S6B ). To test whether IHF stimulated transposition for these systems, we performed experiments in WT and ΔIHF cells for five other systems and found that only two (Tn 7000 and Tn 7014 ) showed a strong IHF dependence ( Figure 5F ). These data suggest that the IHF dependence may not be conserved across all I-F CAST systems, though the level and length of protein overexpression in our transposition assays likely also affect these results.
Given the involvement of IHF and, more generally, the importance of donor/target DNA supercoiling and topology for other mobile elements ( 55 , 56 ), we decided to broadly investigate whether other E. coli NAPs might play a role in transposition. After generating individual knockouts of 5 additional NAP genes ( ycbG , hupA , hupB , hns , and fis ) and measuring integration efficiency within these mutant backgrounds, we found that only the loss of fis affected transposition, decreasing integration efficiency by 2-fold ( Figure S6F ). When we tested the same cohort of NAP knockouts for transposition with the prototypic Tn7 system, IHF had no effect whereas Fis again influenced integration efficiency, though with a ~4-fold increase in the knockout strain ( Figure S7B ). Fis (factor for inversion stimulation) plays diverse roles in altering DNA topology, mediating DNA inversions, and regulating gene expression ( 57 – 59 ); these varied roles, and the lack of a clearly defined consensus sequence, make it difficult to know how Fis impacts transposition in either system, or whether changes in integration efficiency might instead be indirect effects. Interestingly, our amplicon-sequencing detection approach for E. coli Tn 7 transposition also yielded new information about the nature of DNA integration products for the well-studied TnsABCD pathway. Whereas prior studies concluded that TnsD binding defines a single integration site downstream of the essential glmS gene ( 60 – 62 ), we observed surprisingly heterogeneous insertion patterns that sampled a wider sequence space, including rare but reproducible transposition products in the less-common T-LR orientation ( Figure S7C ). These findings highlight the value of deep sequencing to thoroughly and unbiasedly query the range of potential integration products for a given transposable element.
Lastly, we decided to investigate whether IHF might also bias the orientation of transposon integration for CAST systems, since the IHF binding site (IBS) is uniquely present within the transposon left end. After testing bidirectional transposition for two CAST systems in both a WT and ΔIHF strain of E. coli , we found that although the loss of IHF did not affect orientation preference for VchCAST, its loss reversed the dominant orientation for Tn 7000 from T-RL to T-LR ( Figure S6C ). This result raises the intriguing possibility that IHF may be involved in establishing a transpososome architecture that controls the directionality of DNA insertions, at least for some systems. Previous work with the prototypic Tn 7 system found that transposon substrates with two right ends were competent for integration whereas two left ends were not ( 13 ), and we wondered whether a symmetric VchCAST donor with two right ends would similarly be competent for transposition while also eliminating IHF dependency. In agreement with this hypothesis, the loss of IHF had no impact on transposition with a substrate containing two transposon right ends, which was integrated without orientation bias, while a substrate containing two left ends exhibited severely reduced integration efficiency that retained a dependence on IHF ( Figure S6D , E ). Overall, our data support a model ( Figure 5G ) in which IHF binds the region between TBSs L1 and L2 to bend the transposon left end and drive DNA integration, akin to the proposed role of HU in Mu transposition [ 12 ].
RNA-guided DNA integration by CRISPR-associated transposons depends on diverse, sequence-specific nucleic acid determinants. Focusing on VchCAST, a highly efficient and accurate CAST system derived from Vibrio cholerae (also known as Tn 6677 ) ( 24 , 31 ), we employed high throughput screening methods to systematically investigate and characterize these sequence requirements in this study. We first determined the minimal transposon sequences needed for robust activity and validated the importance of each transposase binding site (TBS) found within both left and right ends. Interestingly, our data revealed a broad degree of tolerance to mutagenesis of individual TBSs, a feature corroborated by recent TnsB transposase-DNA structures that show interactions mainly with the DNA backbone rather than specific nucleobases ( 37 – 39 ). The presence of multiple binding sites within each transposon end might allow for accumulative specificity and affinity, and likely play a role in regulating transposition frequency. Our results furthermore suggest that the asymmetric nature of the two transposon ends controls the idiosyncratic preferences of a given element for integrating in one orientation over another.
We uncovered additional regions within the transposon ends that drastically affect integration efficiencies, including a sensitive region within the left end that ultimately revealed a conserved binding site for integration host factor (IHF). Transposition assays with perturbations of the IHF binding site, and in E. coli strains lacking IHF, demonstrated that IHF is critical for efficient transposition of VchCAST and some, but not all, homologous Type I-F CAST systems, at least under the expression conditions we tested. Since IHF is known to facilitate extreme bending of the bound DNA ( 51 , 54 ), we propose that IHF is important for the proper quaternary organization of the transpososome. This hypothesis is further supported by transposon end variants containing alternate spacing between the TBSs, which revealed a conserved periodicity that is consistent with the helical nature of double-stranded DNA. It is striking that, although Type I-F CASTs rely on a multitude of transposon-encoded genes, diverse DNA sequence determinants, and potential additional host-encoded factors, heterologous assays in E. coli with twenty CASTs from a range of gammaproteobacteria revealed active transposition for all ( 18 ). How and why mobile genetic elements would evolve dependencies on host-specific factors are questions that encourage further research into the regulation of transposition and search for additional accessory factors ( 63 ), especially in native host organisms.
We also unbiasedly analyzed sequence biases at the site of integration and found a clear preference for insertions into sites containing a central 5’-YWR-3’ motif, with additional nucleotide preferences 3-bp upstream and downstream of the TSD in regions that appear to make direct contacts with the TnsB transposase from a Type V-K CAST ( 41 ). Remarkably, by projecting this new information onto the integration site patterns we previously obtained for a panel of genomic target sites in E. coli , we were able to explain the observed product heterogeneity, thus enabling guide RNA selection with high predictability for integration products at single-bp resolution. Finally, we exploited our dataset on transposon end mutability and integration site preference to design modified transposon variants that enabled in-frame tagging of endogenous protein-coding genes. In a proof-of-concept experiment, we tagged the endogenous E. coli msrB protein with GFP through modification of a short transposon right end and an in-frame gfp gene, and similar efforts should enable in-frame tagging in other cell types, where transposon end ‘scars’ are converted into functional sequence modifications.
Collectively, our work demonstrates the power of combining rationally designed libraries with deep sequencing approaches. We reveal new insights on the molecular mechanism of RNA-guided transposition while also building a register, at single-bp resolution, of which bases can and cannot be mutated for engineering purposes. These new insights inform future studies of both the biology and application potential of CAST systems.
Supplementary Material
Acknowledgement.
We thank S.R. Pesari for laboratory support; N.E. Sanjana for helpful discussions about pooled library experiments; M.A. Hydorn and J.E. Dworkin for fluorescence microscopy support and microscope access; L.F. Landweber for qPCR instrument access; and the staff at the JP Sulzberger Columbia Genome Center for NGS support.
This research was supported by the National Institutes of Health (Grant numbers DP2HG011650 and R21AI168976 to S.H.S.), the Pew Biomedical Scholars Program (S.H.S.), the Alfred Sloan Foundation Research Fellowship (S.H.S.), the Irma T. Hirschl Career Scientist Award (S.H.S.), and the National Science Foundation (GRFP to M.W.G.W.).
Conflict of interest
Columbia University has filed a patent application related to this work for which M.W.G.W., S.E.K., D.J.Z., and S.H.S. are inventors. M.W.G.W., S.E.K, and S.H.S. are inventors on other patents and patent applications related to CRISPR-Cas systems and uses thereof. M.W.G.W. is a co-founder of Can9 Bioengineering. S.H.S. is a co-founder and scientific advisor to Dahlia Biosciences, a scientific advisor to CrisprBits and Prime Medicine, and an equity holder in Dahlia Biosciences and CrisprBits.
AVAILABILITY
High-throughput sequencing data are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive (BioProject Accession: PRJNA919078). Custom scripts used for analyses of high-throughput sequencing data are available at GitHub ( https://github.com/sternberglab/Walker_Klompe_etal_2023 ). Datasets generated and analyzed in the current study are available from the corresponding authors on reasonable request.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR online.
- 1. Feschotte C. and Pritham E.J. (2007) DNA Transposons and the Evolution of Eukaryotic Genomes. Genetics, 41, 331–368. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. Dubin M.J., Scheid O.M. and Becker C. (2018) Transposons: a blessing curse. Curr Opin Plant Biol, 42, 23–29. [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. Kidwell M.G. and Lisch D.R. (2001) PERSPECTIVE: TRANSPOSABLE ELEMENTS, PARASITIC DNA, AND GENOME EVOLUTION. Evolution, 55, 1–24. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Hickman A.B. and Dyda F. (2015) Mechanisms of DNA Transposition. Microbiol Spectr, 3, MDNA3-0034-2014. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Hickman A.B. and Dyda F. (2016) DNA Transposition at Work. Chem Rev, 116, 12758–12784. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Ivics Z., Hackett P.B., Plasterk R.H. and Izsvák Z. (1997) Molecular Reconstruction of Sleeping Beauty, a Tc1-like Transposon from Fish, and Its Transposition in Human Cells. Cell, 91, 501–510. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Richardson J.M., Dawson A., O’hagan N., Taylor P., Finnegan D.J. and Walkinshaw M.D. (2006) Mechanism of Mos1 transposition: insights from structural analysis. Embo J, 25, 1324–1334. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. Arciszewska L.K. and Craig N.L. (1991) Interaction of the Tn7-encoded transposition protein TnsB with the ends of the transposon. Nucleic Acids Res, 19, 5021–5029. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Ghanim G.E., Rio D.C. and Teixeira F.K. (2020) Mechanism and regulation of P element transposition. Open Biol, 10, 200244. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Hickman A.B., Ewis H.E., Li X., Knapp J.A., Laver T., Doss A.-L., Tolun G., Steven A.C., Grishaev A., Bax A., et al. (2014) Structural Basis of hAT Transposon End Recognition by Hermes, an Octameric DNA Transposase from Musca domestica. Cell, 158, 353–367. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Chen Q., Luo W., Veach R.A., Hickman A.B., Wilson M.H. and Dyda F. (2020) Structural basis of seamless excision and specific targeting by piggyBac transposase. Nat Commun, 11, 3446. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Montaño S.P., Pigli Y.Z. and Rice P.A. (2012) The Mu transpososome structure sheds light on DDE recombinase evolution. Nature, 491, 413–417. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Arciszewska L.K., Drake D. and Craig N.L. (1989) Transposon Tn7 cis-Acting sequences in transposition and transposition immunity. J Mol Biol, 207, 35–52. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Sarnovsky R.J., May E.W. and Craig N.L. (1996) The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products. Embo J, 15, 6348–6361. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Tang Y., Lichtenstein C. and Cotterill S. (1991) Purification and characterisation of the TnsB protein of Tn7: a transposition protein that binds to the ends of Tn7. Nucleic Acids Res, 19, 3395–3402. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Choi K.Y., Spencer J.M. and Craig N.L. (2014) The Tn7 transposition regulator TnsC interacts with the transposase subunit TnsB and target selector TnsD. Proc National Acad Sci, 111, E2858–E2865. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 17. Stellwagen A.E. and Craig N.L. (1998) Mobile DNA elements: controlling transposition with ATP-dependent molecular switches. Trends Biochem Sci, 23, 486–490. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Klompe S.E., Jaber N., Beh L.Y., Mohabir J.T., Bernheim A. and Sternberg S.H. (2022) Evolutionary and mechanistic diversity of Type I-F CRISPR-associated transposons. Mol Cell, 82, 616–628.e5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Petassi M.T., Hsieh S.-C. and Peters J.E. (2020) Guide RNA Categorization Enables Target Site Choice in Tn7-CRISPR-Cas Transposons. Cell, 183, 1757–1771.e18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Mitra R., McKenzie G.J., Yi L., Lee C.A. and Craig N.L. (2010) Characterization of the TnsD-attTn7 complex that promotes site-specific insertion of Tn7. Mobile Dna-uk, 1, 18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Waddell C.S. and Craig N.L. (1989) Tn7 transposition: recognition of the attTn7 target sequence. Proc National Acad Sci, 86, 3958–3962. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Peters J.E., Fricker A.D., Kapili B.J. and Petassi M.T. (2014) Heteromeric transposase elements: generators of genomic islands across diverse bacteria. Mol Microbiol, 93, 1084–1092. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Peters J.E. (2019) Targeted transposition with Tn7 elements: safe sites, mobile plasmids, CRISPR/Cas and beyond. Mol Microbiol, 112, 1635–1644. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Klompe S.E., Vo P.L.H., Halpin-Healy T.S. and Sternberg S.H. (2019) Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature, 571, 219–225. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Strecker J., Ladha A., Gardner Z., Schmid-Burgk J.L., Makarova K.S., Koonin E.V. and Zhang F. (2019) RNA-guided DNA insertion with CRISPR-associated transposases. Science, 365, 48–53. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Saito M., Ladha A., Strecker J., Faure G., Neumann E., Altae-Tran H., Macrae R.K. and Zhang F. (2021) Dual modes of CRISPR-associated transposon homing. Cell, 184, 2441–2453.e18. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Peters J.E., Makarova K.S., Shmakov S. and Koonin E.V. (2017) Recruitment of CRISPR-Cas systems by Tn7-like transposons. Proc National Acad Sci, 114, E7358–E7366. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Faure G., Shmakov S.A., Yan W.X., Cheng D.R., Scott D.A., Peters J.E., Makarova K.S. and Koonin E.V. (2019) CRISPR–Cas in mobile genetic elements: counter-defence and beyond. Nat Rev Microbiol, 17, 513–525. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Halpin-Healy T.S., Klompe S.E., Sternberg S.H. and Fernández I.S. (2020) Structural basis of DNA targeting by a transposon-encoded CRISPR–Cas system. Nature, 577, 271–274. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Hoffmann F.T., Kim M., Beh L.Y., Wang J., Vo P.L.H., Gelsinger D.R., George J.T., Acree C., Mohabir J.T., Fernández I.S., et al. (2022) Selective TnsC recruitment enhances the fidelity of RNA-guided transposition. Nature, 609, 384–393. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Vo P.L.H., Ronda C., Klompe S.E., Chen E.E., Acree C., Wang H.H. and Sternberg S.H. (2021) CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat Biotechnol, 39, 480–489. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Hegde M., Strand C., Hanna R.E. and Doench J.G. (2018) Uncoupling of sgRNAs from their associated barcodes during PCR amplification of combinatorial CRISPR screens. Plos One, 13, e0197547. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Vo P.L.H., Acree C., Smith M.L. and Sternberg S.H. (2021) Unbiased profiling of CRISPR RNA-guided transposition products by long-read sequencing. Mobile Dna-uk, 12, 13. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Feng S., Sekine S., Pessino V., Li H., Leonetti M.D. and Huang B. (2017) Improved split fluorescent proteins for endogenous protein labeling. Nat Commun, 8, 370. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Sharan S.K., Thomason L.C., Kuznetsov S.G. and Court D.L. (2009) Recombineering: a homologous recombination-based method of genetic engineering. Nat Protoc, 4, 206–223. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. McKown R.L., Waddell C.S., Arciszewska L.K. and Craig N.L. (1987) Identification of a transposon Tn7-dependent DNA-binding activity that recognizes the ends of Tn7. Proc National Acad Sci, 84, 7807–7811. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 37. Park J.-U., Tsai A.W.-L., Chen T.H., Peters J.E. and Kellogg E.H. (2022) Mechanistic details of CRISPR-associated transposon recruitment and integration revealed by cryo-EM. Proc National Acad Sci, 119, e2202590119. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Kaczmarska Z., Czarnocki-Cieciura M., Górecka-Minakowska K.M., Wingo R.J., Jackiewicz J., Zajko W., Poznański J.T., Rawski M., Grant T., Peters J.E., et al. (2022) Structural basis of transposon end recognition explains central features of Tn7 transposition systems. Mol Cell, 82, 2618–2632.e7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Tenjo-Castaño F., Sofos N., López-Méndez B., Stutzke L.S., Fuglsang A., Stella S. and Montoya G. (2022) Structure of the TnsB transposase-DNA complex of type V-K CRISPR-associated transposon. Nat Commun, 13, 5792. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 40. Green B., Bouchier C., Fairhead C., Craig N.L. and Cormack B.P. (2012) Insertion site preference of Mu, Tn5, and Tn7 transposons. Mobile Dna-uk, 3, 3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Park J.-U., Tsai A.W.-L., Chen T.H., Peters J.E. and Kellogg E.H. (2022) Mechanistic details of CRISPR-associated transposon recruitment and integration revealed by cryo-EM. Proc National Acad Sci, 119, e2202590119. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 42. Bainton R., Gamas P. and Craig N.L. (1991) Tn7 transposition in vitro proceeds through an excised transposon intermediate generated by staggered breaks in DNA. Cell, 65, 805–816. [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Fayet O., Ramond P., Polard P., Prère M.F. and Chandler M. (1990) Functional similarities between retroviruses and the IS3 family of bacterial insertion sequences? Mol Microbiol, 4, 1771–1777. [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Tang Y., Cotterill S. and Lichtenstein C.P. (1995) Genetic analysis of the terminal 8-bp inverted repeats of transposon Tn7. Gene, 162, 41–46. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Craig N.L., Chandler M., Gellert M., Lambowitz A.M., Rice P.A. and Sandmeyer S.B. (2019) Mobile DNA III. 10.1128/9781555819217. [ DOI ] [ Google Scholar ]
- 46. Mizuuchi K. (1992) Transpositional Recombination: Mechanistic Insights from Studies of Mu and Other Elements. Annu Rev Biochem, 61, 1011–1051. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Lee I. and Harshey R.M. (2001) Importance of the conserved CA dinucleotide at mu termini11Edited by M. Gottesman. J Mol Biol, 314, 433–444. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Zhang Y., Yang J., Yang S., Zhang J., Chen J., Tao R., Jiang Y., Yang J. and Yang S. (2021) Programming Cells by Multicopy Chromosomal Integration Using CRISPR-Associated Transposases. Crispr J, 4, 350–359. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Friedman D.I. (1988) Integration host factor: A protein for all reasons. Cell, 55, 545–554. [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Wang S., Cosstick R., Gardner J.F. and Gumport R.I. (1995) The specific binding of Escherichia coli integration host factor involves both major and minor grooves of DNA. Biochemistry-us, 34, 13082–13090. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. Rice P.A., Yang S., Mizuuchi K. and Nash H.A. (1996) Crystal Structure of an IHF-DNA Complex: A Protein-Induced DNA U-Turn. Cell, 87, 1295–1306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 52. Miller H.I., Kikuchi A., Nash H.A., Weisberg R.A. and Friedman D.I. (1979) Site-specific Recombination of Bacteriophage : The Role of Host Gene Products. Cold Spring Harb Sym, 43, 1121–1126. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Kikuchi A., Flamm E. and Weisberg R.A. (1985) An Escherichia coli mutant unable to support site-specific recombination of bacteriophage λ. J Mol Biol, 183, 129–140. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Swinger K.K. and Rice P.A. (2004) IHF and HU: flexible architects of bent DNA. Curr Opin Struc Biol, 14, 28–35. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Wang Z. and Harshey R.M. (1994) Crucial role for DNA supercoiling in Mu transposition: a kinetic study. Proc National Acad Sci, 91, 699–703. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Chalmers R., Guhathakurta A., Benjamin H. and Kleckner N. (1998) IHF Modulation of Tn10 Transposition: Sensory Transduction of Supercoiling Status via a Proposed Protein/DNA Molecular Spring. Cell, 93, 897–908. [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Schneider R., Lurz R., Lüder G., Tolksdorf C., Travers A. and Muskhelishvili G. (2001) An architectural role of the Escherichia coli chromatin protein FIS in organising DNA. Nucleic Acids Res, 29, 5107–5114. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Bradley M.D., Beach M.B., Koning A.P.J. de, Pratt T.S. and Osuna R. (2007) Effects of Fis on Escherichia coli gene expression during different growth stages. Microbiology+, 153, 2922–2940. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Finkel S.E. and Johnson R.C. (1992) The Fis protein: it’s not just for DNA inversion anymore. Mol Microbiol, 6, 3257–3265. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Kuduvalli P.N., Rao J.E. and Craig N.L. (2001) Target DNA structure plays a critical role in Tn7 transposition. Embo J, 20, 924–932. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Waddell C.S. and Craig N.L. (1988) Tn7 transposition: two transposition pathways directed by five Tn7-encoded genes. Gene Dev, 2, 137–149. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Gay N.J., Tybulewicz V.L. and Walker J.E. (1986) Insertion of transposon Tn7 into the Escherichia coli glmS transcriptional terminator. 234, 111–117. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Schmitz M., Querques I., Oberli S., Chanez C. and Jinek M. (2022) Structural basis for the assembly of the type V CRISPR-associated transposon complex. Cell, 185, 4999–5010.e17. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- View on publisher site
- PDF (4.9 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Early transposon mutagenesis experiments relied on bacteriophages and conjugative bacterial plasmids for the insertion of sequences. These were very non-specific, and made it difficult to incorporate specific genes.
Transposon-insertion sequencing (TIS) methods combine large-scale transposon mutagenesis with next-generation sequencing to estimate the essentiality and/or fitness contribution of each...
Transposon mutagenesis utilizes transposable genetic elements that integrate into a recipient genome to generate random insertion mutations which are easily identified. This forward genetic approach has proven powerful in elucidating complex processes, such as various pathways in methylotrophy.
Transposition can be highly mutagenic, perturbing genome integrity and gene expression in a wide range of organisms. This mutagenic potential has been exploited in the laboratory, where transposons have long been utilized for phenotypic screening and the generation of defined mutant libraries.
In this study, we have developed an efficient and regulatable transposon mutagenesis tool that exploits an IPTG-controlled conditional suicide plasmid. It contains an RSF1010 replicon, an IncQ-type replication origin that allows plasmid replication in most Gram-negative bacteria, as well as a few Gram-positive bacteria [18].
Transposon insertion sequencing (TIS) is a powerful approach that can be extensively applied to the genome-wide definition of loci that are required for bacterial growth under diverse...
Transposon-insertion sequencing (TIS) is a powerful approach that can be widely applied to genome-wide definition of loci that are required for growth in diverse conditions. However, experimental design choices and stochastic biological processes ...
The combination of transposon mutagenesis with next-generation sequencing has emerged as a useful tool for identifying putative gene function in a high-throughput manner.
Here, we report the development of a robust and inexpensive High-Throughput Transposon Mutagenesis (HTTM) protocol and validate the method using Escherichia coli strain BW25113, the parental strain of the KEIO collection.
Here, we exploit pooled library screening and high-throughput sequencing to reveal novel sequence determinants during transposition by the Type I-F Vibrio cholerae CAST system.